A facile synthesis of glycerol carbonate by transesterification of glycerol with dimethyl carbonate: Ceria based catalysts
DOI:
https://doi.org/10.29356/jmcs.v62i4.859Keywords:
Glycerol, Ceria/Zirconia/Magnesia, Glycerol carbonate, TransesterificationAbstract
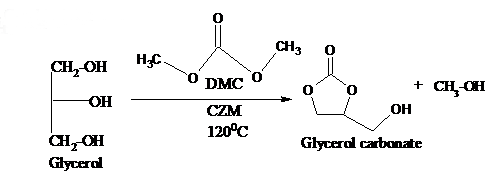
Abstract. Solid base catalysts such as Ceria-Zirconia-Magnesia with different mole ratio of magnesium were prepared by impregnation method and characterized by CO2-TPD, PXRD, FT-IR and ICP-OES analysis. The catalytic activity of the catalysts was tested in the liquid phase transesterification of glycerol with dimethyl carbonate to synthesise glycerol carbonate. Optimization of reaction condition was carried out by varying the molar ratio of the reactants, temperature and time. The highest yield (97 %) of glycerol carbonate was obtained at a reactant molar ratio of 1:3 at 120 °C in 6 h. Study of reusability and reactivation of solid base catalyst was also taken up. A suitable base catalysed mechanism for the formation of glycerol carbonate is proposed.
Resumen. Se prepararon catalizadores de base sólida como Ceria-Zirconia-Magnesia con diferentes proporciones molares de magnesio mediante el método de impregnación y se caracterizaron por análisis de CO2-TPD, PXRD, FT-IR e ICP-OES. La actividad catalítica de los catalizadores se probó en la transesterificación en fase líquida de glicerol con carbonato de dimetilo para sintetizar carbonato de glicerol. La optimización de las condiciones de reacción se llevó a cabo variando la relación molar de los reactivos, la temperatura y el tiempo. El mayor rendimiento (97 %) de carbonato de glicerol se obtuvo a una relación molar reactiva de 1:3 a 120 °C en 6 h. También se realizó un estudio de la reutilización y reactivación del catalizador de base sólida. Se propone un mecanismo catalítico básico adecuado para la formación de carbonato de glicerol.
Downloads
References
Marakatti, S, V.; Anand Halgeri B.; RSC Adv, 2015, 5, 14286-14293. DOI: https://doi.org/10.1039/C4RA16052E
Tanb, Y. Z.; Liu, J.; Wang, Kang, M.; Yin, N.; Wang, X.; Zhub, Y.; J. Braz. Chem. Soc, 2014, 25, 152-160.
Burk, R. M.; Roof, M. B.; Tetrahedron Lett, 1993, 34, 395-398. DOI: https://doi.org/10.1016/0040-4039(93)85085-B
Mizuno, T.; Nakai, T.; Mihara, M.; Heteroat. Chem, 2010, 21, 99-102. DOI: https://doi.org/10.1002/hc.20583
Hu, J.; Li, J.; Gu, Y.; Guan, Z.; Ni, W.; Li, Y.; Li, T.; Appl. Catal. A: Gen, 2010, 386, 188- 193. DOI: https://doi.org/10.1016/j.apcata.2010.07.059
Vieville, C.; Yoo, J.; Pelet, S.; Mouloungui, Z.; Catal. Lett, 1998, 56, 245-247. DOI: https://doi.org/10.1023/A:1019050205502
George, J.; Patel, Y.; Pillai, S.M.; Munshi, P.; J. Mol. Catal. A: Chem, 2009, 304, 1-7. DOI: https://doi.org/10.1016/j.molcata.2009.01.010
Climent, M.J.; Corma, A.; Frutos, P. D.; Iborra, S.; Noy, M.; Velty, A.; J. Catal, 2010, 269, 140-149. DOI: https://doi.org/10.1016/j.jcat.2009.11.001
Hammond, C.; Lopez-Sanchez, J. A.; Rahim, M. H. A; Dimitratos, N.; Jenkins, R. L.; Carley, A. F.; He, Q.; Kiely C. J.; Knight, D.W.; Hutchings, G.J.; Dalton Trans, 2011, 40, 3927-3937 DOI: https://doi.org/10.1039/c0dt01389g
Ochoa-Gómez, J.R; Gómez-Jiménez-Aberasturi, O.; Maestro Madurga, B.; Pesquera- Rodríguez A.; Ramírez López, A. C.; Lorenzo-Ibarreta L.; Torrecilla-Soria, J.; Villarán-Velasco, M. C.; Appl. Catal. A: Gen, 2009, 366, 315-324.
Bell, J. B.; US Pat, 1959, 2, 915 -529.
Alvarez, M. G.; Segarra, A. M.; Contreras, S.; Sueiras, J. E.; Medina, F.; Figueras, F.; Chem. Eng. J, 2010, 161, 340-345. DOI: https://doi.org/10.1016/j.cej.2009.12.036
Bai, R.; Wang, Y.; Wang, S.; Mei, T.; Li, G.; Li, Fuel Process. Technol, 2010, 106, 209-214. DOI: https://doi.org/10.1016/j.fuproc.2012.07.027
Bancquart, S.; Vanhove, C.; Pouilloux, Y.; Barrault, J.; J. Appl. Catal. A: Gen, 2001,218, 1-11 DOI: https://doi.org/10.1016/S0926-860X(01)00579-8
Sato, S.; Sato, F.; Gotoh, H.; Yamada, Y.; ACS Catal, 2013, 3, 721–734. DOI: https://doi.org/10.1021/cs300781v
Venkatesh, Mohammed Shamshuddin, S. Z.; Shyamsundar, M.; Vasanth, V. T.; J. Mex. Chem. Soc, 2014, 58, 378-385.
Gotoh, H.; Yamada, Y.; Sato, S.; Appl. Catal. A: Gen, 2010, 377, 92-98. DOI: https://doi.org/10.1016/j.apcata.2010.01.025
Sakata, Y.; Ponec, V.; Appl. Catal. A: Gen, 1998, 166, 173-184. DOI: https://doi.org/10.1016/S0926-860X(97)00252-4
Kobune, M.; Sato, S.; Takahashi, R.; J. Mol. Catal. A: Chem,2008, 279, 10-19. DOI: https://doi.org/10.1016/j.molcata.2007.09.027
Rathod, S.; Arbad, B.; Lande, M.; Chinese J of Catal, 2010, 31, 631-636. DOI: https://doi.org/10.1016/S1872-2067(09)60078-4
Trovarelli, A.; catalysis by ceria and related materials London, 2013, 12, 1-888. DOI: https://doi.org/10.1142/p870
Rathod, B. S.; Machhinndra, K.; Lande, R.; Balasaheb, Arbad, Gambhire, A.; Arabian J of Chem, 2014, 7, 373-378.
Hussein, A.; Khalaf, Springer plus, 2013, 2:619, 1-9.
Parameswaram, G.; Srinivas, M.; HariBabu, B.; Sai Prasad, P. S.; Lingaiah, N.; Catal. Sci. Technol, 2013, 3, 3242-3249. DOI: https://doi.org/10.1039/c3cy00532a
Malyaadri, M.; Jagadeeswaraiah, K.; Sai Prasad P. S.; Lingaiah, N.; Catalysts. Appl.Catal. A: Gen, 2011, 401, 153-157. DOI: https://doi.org/10.1016/j.apcata.2011.05.011
Peterson Santos Querino, Jose Renato Carlos Bispo, Maria do Carmo Rangel, Catalysis Today, 2005, 107, 930-925. DOI: https://doi.org/10.1016/j.cattod.2005.07.032
Rathoda, S.; Navgireb, M.; Arbadb, B.; Lande, M. S.; Afr. J. Chem, 2012, 65, 196-201.
Hattori H.; Chem Rev, 1995, 95, 537-558. DOI: https://doi.org/10.1021/cr00035a005
Tsuji H.; Hattori H.; Catal Today, 2006, 116, 239-243. DOI: https://doi.org/10.1016/j.cattod.2006.01.034
Okoye, P. U.; Abdullah, Z. B.; Hameed, H.; J. Taiwan Institute of Chem. Eng, 2016, 68, 51-58. DOI: https://doi.org/10.1016/j.jtice.2016.09.011
Zheng, L.; Xia, S.; Lu, X. Z.; Hou, Z.; Chinese. J. Catal, 2015, 36, 1759-1765. DOI: https://doi.org/10.1016/S1872-2067(15)60915-9
Khayoon, M.S.; Hameed, B.H.; Appl Catal A Gen, 2013, 466, 272-281. DOI: https://doi.org/10.1016/j.apcata.2013.06.044
Prakruthi, H. R.; Jai Prakash, B. S.; Bhat, Y. S.; J. Mol. Catal. A: Chem, 2015, 408, 214-220. DOI: https://doi.org/10.1016/j.molcata.2015.07.036
Liu, J.; Li, Y.; Zhang, J.; He, D. App Catal A: Gen, 2016, 513. 9-18. DOI: https://doi.org/10.1016/j.apcata.2015.12.030
Cinzia Chiappe.: Sunita Rajamani: Pure Appl. Chem, 2012, 84, 755–762. DOI: https://doi.org/10.1351/PAC-CON-11-07-06
Yin Wang: Chunling Liu: Jihong Sun: Rongzhen Yang: Wensheng Dong.: Sci. china chem., 2015, 58, 708–71. DOI: https://doi.org/10.1007/s11426-014-5173-0
Jose, R.; Gomez, C.; Go, O. Aberasturi, M. N. J.; Madurga, B. M.; Rodr?guez, A. P.; Lopez, C. R.; Ibarreta, L. L.; Soria, J. T.; Velasco, C. M. V.; App Catal A: Gen, 2009, 366, 315-324. DOI: https://doi.org/10.1016/j.apcata.2009.07.020

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









