UV Spectrophotometric Determination of Thermodynamic Dissociation Constants of Some Aromatic Hydrazones in Acid Media
DOI:
https://doi.org/10.29356/jmcs.v63i4.794Keywords:
p-nitro-p-substituted benzoylhydrazones, dissociation constants, protonation, UV spectroscopy, semiempirical AM1 and PM3 methodsAbstract
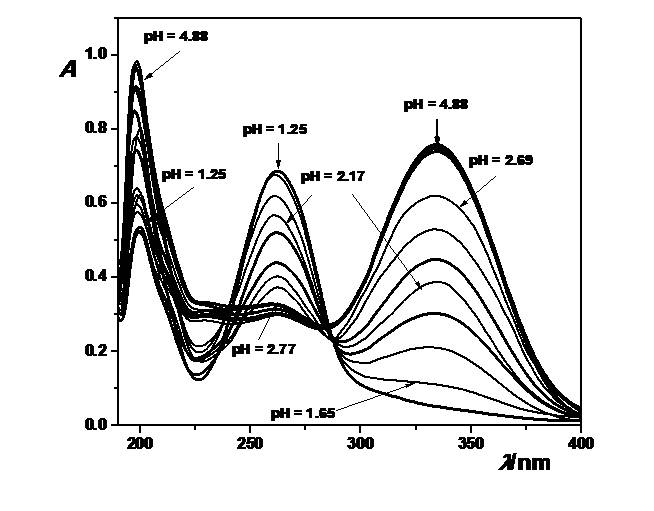
The spectral behavior of some p-nitro-p-substituted benzoylhydrazones in the perchloric acid media was followed, applying the UV spectroscopy. The position of the absorption maximum in the spectra was defined in acidic media and the electronic transitions were discussed, as well (7<pH<1). The equilibrium between neutral and protonated form was investigated in the ethanol-water (V/V, 1:1) solutions. The observed changes in the UV spectra suggested that protonation process took place in one step. The pH region of protonation ranges between 1.4 and 2.9. Using the changes in the UV spectra which appear as a result of the protonation reaction the stoichiometric dissociation constants were determined numerically (pKBH+ = n·pH + logI) and graphically (intercept of the dependence of logI on pH). Thermodynamic dissociation constants were estimated as an intercept of dependence of pKBH+ on square root of the ionic strength. In order to achieve that, measurements were performed at different ionic strengths: 0.1, 0.25 and 0.5 mol/dm3, adjusted with sodium perchlorate. The obtained thermodynamic pKBH+ values ranged between 2.07 and 2.58. In order to predict proton transfer at a given pH, semiempirical methods AM1 and PM3 were applied. The influence of the substituents present in the p-position of the benzene ring on pKBH+ values of investigated hydrazones was discussed, too. Total energy, binding energy, enthalpy of formation, Gibbs energies of formation, atomic charge and proton affinity values were used to predict protonation site in hydrazone molecule. Furthermore, the stability and the proton affinity of the isomers (E and Z) in which hydrazones exist and their protonated forms were defined.
Downloads
References
Brehme, R.; Enders, D.; Fernandez R.; Lassaletta, J. M. Eur. J. Org. Chem. 2007, 34, 5629-5660. DOI: https://doi.org/10.1002/ejoc.200700746
Belskaya, N. P.; Dehaen W.; Bakulev, V. A. ARKIVOC, 2010, (i), 275-332. DOI: https://doi.org/10.3998/ark.5550190.0011.108
Banerjee, S.; Mondal, S.; Chakraborty, W.; Sen, S.; Gachhui, R.; Butcher, R. J.; Slawin, A. M. Z.; Mandal C.; Mitra, S. Polyhedron, 2009, 28(13), 2785-2793. DOI: https://doi.org/10.1016/j.poly.2009.05.071
Mao, J.; Wang, Y.; Wan, B.; Kozikowski A. P.; Franzblau, S. G. Chem. Med. Chem. 2007, 2(11), 1624-1630. DOI: https://doi.org/10.1002/cmdc.200700112
Ali, Md. R.; Marella, A.; Alam, Md. T.; Naz, R.; Akhter, M.; Shaquiquzzaman, Md.; Saha R.; Tanwar, O.; Alam Md. M.; Hooda, J. Indonesian J. Pharm., 2012, 23, 193-202.
Kumar, P.; Rai, A.; Singh, M.; Kumar, D.; Sahdev A. K.; Raj V. EC Pharm. Sci. 2016, 23, 278 (2016). DOI: https://doi.org/10.1016/B978-0-12-803640-2.00002-6
Kumar, N.; Chauhan, L. S.; Dashora N.; Sharma, C. S. Sch. Acad. J. Pharm., 2014, 3(5), 366-373.
Padmini, K.; Jaya Preethi, P.; Divya, M.; Rohini, P.; Lohita, M. Swetha K. Kaladar, P. Int. J Pharm. Res. & Rev. 2013, 2(8), 43-58.
Rollas S.; Küçükgüzel, S.G. Molecules, 2017, 12, 1910-1939. DOI: https://doi.org/10.3390/12081910
Singh, R. B.; Jain P.; Singh, R. P. Talanta, 1982, 29, 77-84. DOI: https://doi.org/10.1016/0039-9140(82)80024-6
Suvarapu, L. N.; Seo1, Y. K.; Baek S. O.; Ammireddy, V. R. E-Journal of Chemistry, 2012, 9(3), 1288-1304. DOI: https://doi.org/10.1155/2012/534617
Naskar, S.; Naskar, S.; Mondal, S.; Majhi, P. K.; Drew M. G. B.; Chattopadhyay, S. K. Inorg. Chim. Acta, 2011, 371, 100-106. DOI: https://doi.org/10.1016/j.ica.2011.03.051
Singh M.; Raghav, N. Int. J. Pharm. Pharm. Sci. 2011, 3(4), 26-32.
Avila Terra, L. H. S.; da Cunha Areias M. C.; Gaubeur, I.; Encarnacion M.; Suarez-iha V. Spectros. Lett. 1999, 32, 257-271. DOI: https://doi.org/10.1080/00387019909349981
Mohan, M.; Gupta, M. P.; Chandra L.; Jha, N. K. Inorg. Chim. Acta, 1988, 151, 61-68 DOI: https://doi.org/10.1016/S0020-1693(00)83485-4
Aggarwal, N.; Kumar, R.; Srivastva, C.; Dureja P.; Khurana, J. M. J. Agric. Food Chem. 2010, 58, 2056-3061. DOI: https://doi.org/10.1021/jf904144e
Liu, M.; Wang, Y.; Wangyang, W. S. Z.; Liu, F.; Cui Y. L.; Duan, Y. S. J Agric Food Chem. 2010, 58, 6859-6863. DOI: https://doi.org/10.1021/jf1000919
Pathare, B.; Tambe, V.; Dhole S.; Patil, V. Int. J. Pharm. 2014, 4(1), 278-285.
Beckett A. H.; Stenlake, J. B. Practical pharmaceutical chemistry. 4th Edition – part one, CBS publishers and distributors PVT LTD, New Delhi, 1997, 88-120.
Brahmankar D. M.; Jaiswal, S. B. Biopharmaceutics & pharmacokinetics. 2nd Ed., Vallabh prakashan, Delhi, 2009, 42-60.
Zalewski R. I.; Géribaldi, S. J. Chem. Soc., Perkin Trans. 1988, 2, 113-115. DOI: https://doi.org/10.1039/P29880000113
Garcia, B.; Casado, R. M.; Castillo, J.; Ibeas, S.; Domingo, I.; Leal, J. M. J. Phys. Org. Chem., 1993, 6, 101-106. DOI: https://doi.org/10.1002/poc.610060206
Dewar M. J. S.; Dieter, K. M. J. Am. Chem. Soc. 1986, 108, 8075-8086. DOI: https://doi.org/10.1021/ja00285a033
Steawart, J. J. P. J. Comput. Chem. 1989, 10, 209-216. DOI: https://doi.org/10.1002/jcc.540100208
Ienascu, I. M. C.; Lupea, A. X.; Popescu, I. M.; Padure M. A.; Zamfir, A. D. J. Serb. Chem. Soc. 2009, 74, 847-855. DOI: https://doi.org/10.2298/JSC0909847I
Rajput A. P.; Rajput, S. S. Int. J Pharm. Tech. Research, 2009, 1(4), 1605-1611.
Jankulovska, M.; ?olan?eska-Ra?enovi?, K.; Dimova, V.; Spirevska I.; Makreski P. Org. Chem., An Ind. J. 2012, 8, 326-334.
Jankulovska, M.; Spirevska I.; Ragenovi?, K. ?. Bull. Chem. Technol. Macedonia, 2006, 25, 29-37.
Davis C. T.; Geissman, T. A. J. Am. Chem. Soc. 1954, 76, 3507-3511. DOI: https://doi.org/10.1021/ja01642a045
E. J. King, Acid-Base Equilibria, Pergamon Press, Oxford, 1965, 1.
Dewar, M. J. S.; Zoebisch, E. G.; Healy E. F.; Stewart, J. J. P. J. Am. Chem. Soc. 1985, 107, 3902-3909. DOI: https://doi.org/10.1021/ja00299a024
Kireev, V. A. Methods of Practical Calculations in Thermodynamics of Chemical Reactions, Khimiya, Moscow, 1975, 1.
Kristallovich, E. L.; Eshimbetov, A. G.; Chuvylkin, V. D.; Belenkii L. I.; Shakhidoyatov, Kh. M. Chem. Nat. Compd. 2003, 39(5), 495-500. DOI: https://doi.org/10.1023/B:CONC.0000011127.28348.ca
Echevarria, A.; Nascimento, M.; Gerônimo, V.; Miller J.; Giesbrecht, A. J. Braz. Chem. Soc. 1999, 10(1), 60-64. DOI: https://doi.org/10.1590/S0103-50531999000100010
Amrallah, A. H.; Abdalla N. A.; Haty, E. El. J. Chin. Chem. Soc. 2006, 53, 697-706. DOI: https://doi.org/10.1002/jccs.200600092
Jankulovska M. S.; Spirevska, I.; Maced. J. Chem. Chem. Eng. 2014, 33(1), 85-96. DOI: https://doi.org/10.20450/mjcce.2014.370
Perisi?-Janji?, N. U.; Lazarevi?, M.; Janji?, J.; Klisareva, Lj.; U. Scientist Phyl. Sciences. 1995, 7, 64-68.
Gaubeur, I.; Vincenza, M. R.; Iha, K.; Vázquez, M. E. S. I.; Eclética química. 2000, 25, 63-76. DOI: https://doi.org/10.1590/S0100-46702000000100006

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









