Hybrid functionalized phosphonate silica: insight into chromium removal chemistry from aqueous solutions
DOI:
https://doi.org/10.29356/jmcs.v63i2.793Keywords:
amorphous silica, sol-gel process, Cr(III) ions, NMR, XPSAbstract
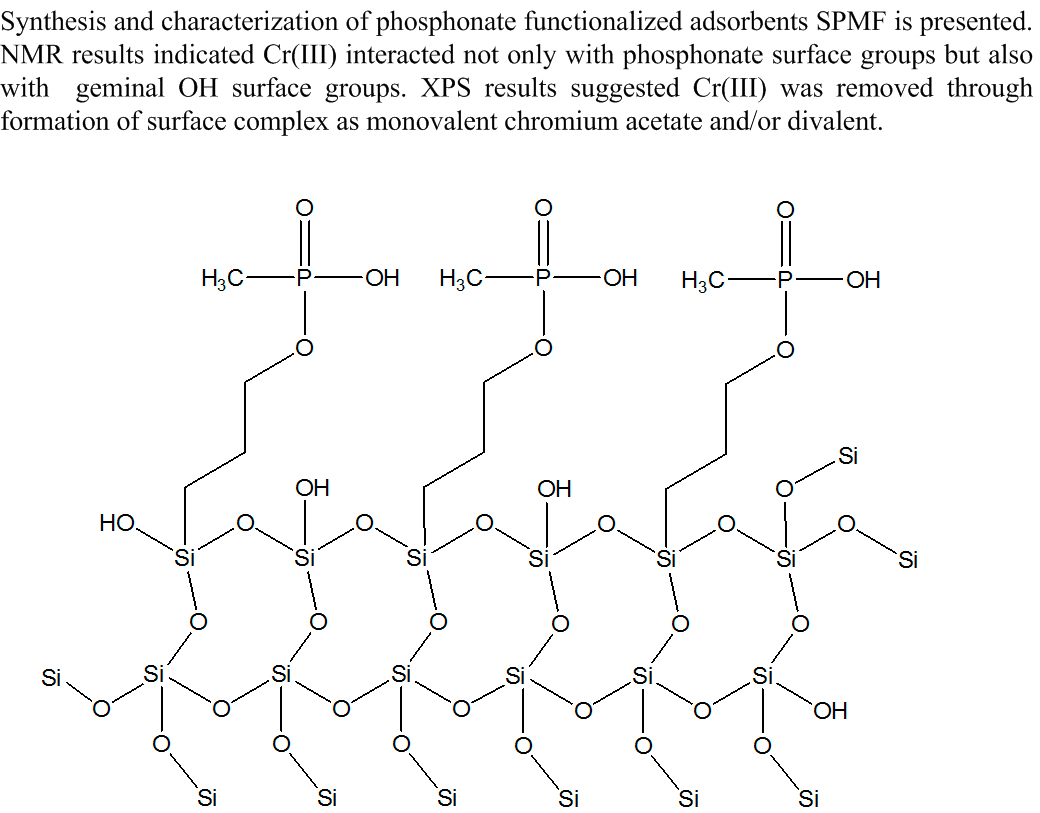
Abstract. Insight into Cr(III) ions removal chemistry from aqueous solutions was gained by using hybrid phosphonate-functionalized silica adsorbents synthesized through a modified route of sol-gel processing (SPMF). Evaluation of the degree of metal removal was obtained from kinetics and batch experiments. Elemental analysis, FTIR, NMR and XPS techniques were used to study the nature of surface complex formed on adsorbent. Adsorption equilibrium results showed a maximum Cr(III) removal of 78.639 mg g-1Cr(III) at pH 3.6 on adsorbent SPMF04; kinetics measurements indicated that equilibrium was reached in 80 min contact time. The achievement of 2.923 mmol P/g as phosphonate groups was obtained. A Langmuir-type mechanism explained the adsorption equilibrium results whereas kinetic measurements were explained through a pseudo-second order mechanism. FTIR measurements indicated a strong influence of Cr(III) adsorbed on surficial functional groups. 29Si CP MAS NMR results indicated that Cr(III) interacted not only with phosphonate surface groups but also with a large of amount of geminal OH surface groups. XPS studies suggested that Cr(III) was removed through the formation of the surface complex R as monovalent chromium acetate and/or divalent. The adsorbent SPMF04 can be potentially employed in industrial applications.
Resumen. Información sobre la química de remoción de Cr(III) de soluciones acuosas fue obtenida usando adsorbentes de sílice hibrida funcionalizada con fosfonato sintetizados a través de una ruta modificada del proceso sol-gel (SPMF). La evaluación del grado de remoción de metal fue obtenida a partir de experimentos de cinética y por lotes. Técnicas de análisis elemental, FTIR, NMR y XPS fueron usadas para estudiar la naturaleza del complejo de superficie formado sobre el adsorbente. Los resultados del equilibrio de adsorción mostraron una remoción máxima de Cr(III) de 78.639 mg g-1 Cr(III) a pH 3.6 sobre el adsorbente SPMF04; las mediciones cinéticas indicaron que el equilibrio fue alcanzado en 80 min de tiempo de contacto. El logro de 2.923 mmol P/g como grupos fosfonato fue obtenido. Un mecanismo tipo Langmuir explicó los resultados del equilibrio de adsorción mientras que las mediciones cinéticas fueron explicadas a través de un mecanismo de pseudo-segundo orden. Los resultados de 29Si CP MAS NMR indicaron que Cr (III) interactuó no solamente con grupos fosfonato de superficie sino también con una gran cantidad de grupos OH de superficie geminales. Los estudios de XPS sugirieron que Cr(III) fue removido a través de la formación del complejo de superficie R como acetato de cromo monovalente y/o divalente. El adsorbente SPMF04 puede ser potencialmente empleado en aplicaciones industriales.
Downloads
References
L. Khezami and R. Capart. J. Hazard. Mater., 2005, 123, 1, 223–231. DOI: https://doi.org/10.1016/j.jhazmat.2005.04.012
P. A. Kobielska, A. J. Howarth, O. K. Farha, and S. Nayak. Coord. Chem. Rev., 2018, 358, 92–107. DOI: https://doi.org/10.1016/j.ccr.2017.12.010
P. Taylor, I. Narin, M. Soylak, K. Kayakirilmaz, L. Elci, and M. Dogan. Anal. Lett., 2014, 35, 37–41.
G. Bayramoglu and M. Y. Arica. J. Hazard. Mater., 2011,187, 1–3, 213–221. DOI: https://doi.org/10.1016/j.jhazmat.2011.01.022
B. Gordon, P. Callan, and C. Vickers, 2008, WHO Chron., 38, 3, 564.
T. N. De Castro Dantas, A. A. D. Neto, and M. C. P. De A. Moura. Water Res., 2001, 35, 9, 2219–2224. DOI: https://doi.org/10.1016/S0043-1354(00)00507-8
M. Costa. Toxicology and Applied Pharmacology, 2003, 188,1, 1–5. DOI: https://doi.org/10.1016/S0041-008X(03)00011-5
M. Ezoddin, F. Shemirani, and R. Khani. Desalination, 2010, 262, 1–3, 183–187. DOI: https://doi.org/10.1016/j.desal.2010.06.007
A. M. Farag, May, T., Marty, G. D., Easton, M., Harper, D. D., Little, E. E., Cleveland, L. Aquat. Toxicol., 2006, 76, 3–4, 246–257. DOI: https://doi.org/10.1016/j.aquatox.2005.09.011
D. E. Kimbrough, Y. Cohen, A. M. Winer, L. Creelman, and C. Mabuni. Critical Reviews in Environmental Science and Technology, 1999, 29, 1, 1–46. DOI: https://doi.org/10.1080/10643389991259164
S. Kocaoba and G. Akcin. Adsorption, 2003, 9, 2, 143–151. DOI: https://doi.org/10.1023/A:1024293310492
A. Puri and M. Kumar. Indian J. Occup. Environ. Med., 2012, 16, 1, 40. DOI: https://doi.org/10.4103/0019-5278.99696
[A. Duran, M. Tuzen, and M. Soylak. Food Chem. Toxicol., 2011, 49, 7,1633–1637. DOI: https://doi.org/10.1016/j.fct.2011.04.016
Report EPA 815-R-03-002, Washington, DC., 2003.
C. Földi, R. Dohrmann, K. Matern, and T. Mansfeldt. J. Soils Sediments, 2013, 13, 7, 1170–1179. DOI: https://doi.org/10.1007/s11368-013-0714-2
D. Dias, N. Lapa, M. Bernardo, W. Ribeiro, I. Matos, I. Fonseca, F. Pinto. Bioresource Technol. 266, 2018, 139-150. DOI: https://doi.org/10.1016/j.biortech.2018.06.054
S. Sainia, Simarpreet Arora, Kirandeep, Bhupinder Pal Singh, Jatinder Kaur Katnoria, Inderpreet Kaur. J Environ Chem Eng. 6, 2, 2018, 2965-2974. DOI: https://doi.org/10.1016/j.jece.2018.04.045
E. Aranda-García,Eliseo Cristiani-Urbina. Environ Sci Pollut Res, 2019, 26:3157–3173 DOI: https://doi.org/10.1007/s11356-017-0248-z
L. Ayele, Eduardo Pérez, Álvaro Mayoral, Yonas Chebude, Isabel Díaz. J Chem Technol Biotechnol. 93, 1, 2018, 146-154. DOI: https://doi.org/10.1002/jctb.5334
V. Shojaei, Hamid Khoshdast. Physicochem. Probl. Miner. Process., 2018, 54(3), 1014-1025.
S. Vasudevan, Lakshmi, J., Sozhan, G. Clean-Soil Air Water. 2009, 37, 45–51. DOI: https://doi.org/10.1002/clen.200800175
E. Bazrafshan, Moein, H., Mostafapour, F.K., Nakhaie, S. J. Chem. 2013, 2013, 640139. DOI: https://doi.org/10.1155/2013/640139
O. Sahu, Mazumdar, B., Chaudhari, P.K. Environ. Sci. Pollut. Res. 2014, 21, 2397–2413. DOI: https://doi.org/10.1007/s11356-013-2208-6
J. Kyzio?-Komosi?ska, Joanna Augustynowicz, Wojciech Lasek, Justyna Czupio?, Daniel Oci?ski, J. Environ. Manage. 214, 2018, 295-304. DOI: https://doi.org/10.1016/j.jenvman.2018.03.010
R. O. Ogbodu, Martins O. Omorogie, Emmanuel I. Unuabonah, Jonathan O. Babalola. Environ Prog Sustain Energy. 34,6, 2015, 1694-1704. DOI: https://doi.org/10.1002/ep.12175
A. Shukla, S. Srivastava, S. F. D’Souza. Int. J. Environ. Sci. Technol. 2018, 15:2701–2712. DOI: https://doi.org/10.1007/s13762-018-1766-z
C. A. Quirarte-Escalante, V. Soto, W. De La Cruz, G. R. Porras, R. Manríquez, and S. Gomez-Salazar. Chem. Mater., 2009, 21, 8, 1439–1450. DOI: https://doi.org/10.1021/cm801480v
S. E. Gomez-Gonzalez, G. G. Carbajal-Arizaga, R. Manriquez-Gonzalez, W. De la Cruz-Hernandez, S. Gomez-Salazar, Mater. Res. Bull., 2014, 59, 394–404. DOI: https://doi.org/10.1016/j.materresbull.2014.07.035
P. Yin, Tian, Y., Wang, Z., Qu, R., Liu, X., Xu, Q., Tang, Q. Mater. Chem. Phys., 2011, 129, 1–2, 168–175. DOI: https://doi.org/10.1016/j.matchemphys.2011.03.067
Y. Liu, L. Guo, L. Zhu, X. Sun, and J. Chen. Chem. Eng. J., 2010, 158, 2, 108–114. DOI: https://doi.org/10.1016/j.cej.2009.12.012
J. S. Lee and L. L. Tavlarides. Solvent Extr. Ion Exch. 2002, 20, 3, 407–427. DOI: https://doi.org/10.1081/SEI-120004814
J. S. Lee, S. Gomez-Salazar, and L. L. Tavlarides, React. Funct. Polym., vol. 49, no. 2, pp. 159–172, 2001. DOI: https://doi.org/10.1016/S1381-5148(01)00071-2
U. Schubert, N. Hiising, and A. Lorenz. Chem. Mater., 1995, 7, 11, 2010–2027. DOI: https://doi.org/10.1021/cm00059a007
M. Streat. React. Funct. Polym., 1998, 38, 219–226. DOI: https://doi.org/10.2166/wst.1998.0212
B. Liu, Y. Fang, and M. Terano. J. Mol. Catal. A Chem., 2004, 219, 1, 165–173. DOI: https://doi.org/10.1016/j.molcata.2004.05.001
C. Brinker and G. Scherer. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing, 1990.
J. S. Lee and L. L. Tavlarides. New York: 14, Ch. 5. Marcel Dekker, 2001.
M. Thommes, Kaneko, K., Neimark, A. V, Olivier, J. P., Rodriguez-reinoso, F., Rouquerol, J., Sing, K. S. W. IUPAC Technical Report, 87, 1051–1069, 2015. DOI: https://doi.org/10.1515/pac-2014-1117
L. L. Hench and J. K. West. Chem. Rev., 1990, 90, 1, 33–72. DOI: https://doi.org/10.1021/cr00099a003
R. Deshpande, D.-W. Hua, D. M. Smith, and C. J. Brinker. J. Non. Cryst. Solids, 1992, 144, 32–44. DOI: https://doi.org/10.1016/S0022-3093(05)80380-1
H. Chen and A. Wang. J. Hazard. Mater., 2009, 165, 1–3, 223–231. DOI: https://doi.org/10.1016/j.jhazmat.2008.09.097
Y. Niu, Qu, R., Chen, H., Mu, L., Liu, X., Wang, T.,Sun, C. J. Hazard. Mater., 2014, 278, 267–278. DOI: https://doi.org/10.1016/j.jhazmat.2014.06.012
S. Sreesai and S. Sthiannopkao. Can. J. Civ. Eng., 2009, 719, 709–719. DOI: https://doi.org/10.1139/L09-008
J. Zhang, Zhai, S., Li, S., Xiao, Z., Song, Y., An, Q., Tian, G. Chem. Eng. J., 2013, 215–216, 461–471. DOI: https://doi.org/10.1016/j.cej.2012.11.043
P. X. Sheng, Y.-P. Ting, and J. P. Chen. Ind. Eng. Chem. Res., 2007, 46, 8, 2438–2444. DOI: https://doi.org/10.1021/ie0615786
G. Socrates. Infrared and Raman characteristic group frequencies. https://doi.org/10.1002/jrs.1238
D. L. Pavia, G. M. Lampman, and G. S. Kriz, Harcourt College Publishers, 2001.
D. J. K. Robert M. Silverstein, Francis X. Webster, Spectrometric identification of organic compounds. 2005.
D. A. Skoog, F. J. Holler, T. A. Nieman, and M. C. M. Gómez, McGraw-Hill, 2000.
J.-P. Mercier, P. Morin, M. Dreux, and A. Tambute. Chromatographia, 1998, 48, 7, 529–534. DOI: https://doi.org/10.1007/BF02466645
H.-F. Klein. Angew. Chemie Int. Ed., 2005, 44, 45, 7331. DOI: https://doi.org/10.1002/anie.200485333
S. Deshpande, S. Dakshinamurthy, S. C. Kuiry, R. Vaidyanathan, Y. S. Obeng, and S. Seal. Thin Solid Films, 2005, 483, 1–2, 261–269. DOI: https://doi.org/10.1016/j.tsf.2004.12.063
M. A. Sharif and H. Sueyoshi. Ceram. Int., 2009, 35, 1, 349–358,. DOI: https://doi.org/10.1016/j.ceramint.2007.11.002
K. Idczak, P. Mazur, L. Markowski, M. Ski?cim, and M. Musia?. Applied Surface Science, 2012, 258, 21, 8349–8353. DOI: https://doi.org/10.1016/j.apsusc.2012.02.125
B. Zhang, T. Kong, W. Xu, R. Su, Y. Gao, and G. Cheng. Langmuir, 2010, 26, 6, 4514–4522,. DOI: https://doi.org/10.1021/la9042827
A. Viinikanoja, J. Lukkari, T. Ääritalo, T. Laiho, and J. Kankare. Langmuir, 2003, 19, 7, 2768–2775. DOI: https://doi.org/10.1021/la0266738
V. Zoulalian, S. Zürcher, S. Tosatti, M. Textor, S. Monge, and J. J. Robin. Langmuir, 2010, 26, 1, 74–82. DOI: https://doi.org/10.1021/la902110j
D. R. Wheeler and O. D. Faut. Appl. Surf. Sci., 1984, 18, 1–2, 106–122. DOI: https://doi.org/10.1016/0169-4332(84)90040-0
M. F. Beaux, N. J. Bridges, M. Dehart, T. E. Bitterwolf, and D. N. McIlroy. Appl. Surf. Sci., 2011, 257, 13, 5766–5771. DOI: https://doi.org/10.1016/j.apsusc.2011.01.097
M. (1980). Brückner, R., Chun, H.-U., Goretzki, H., Sammet. Journal of Non-Crystalline Solids, 1980, 42, 49–60. DOI: https://doi.org/10.1016/0022-3093(80)90007-1
[J. Stypula, B., Stoch. Corros. Sci., 1994, 36, 12, 2159–2167. DOI: https://doi.org/10.1016/0010-938X(94)90014-0
[C. D. Wagner, W. M. Riggs, L. E. Davis, and J. F. Moulder, Handbook of X-Ray Photoelectron Spectroscopy. 1979.
H. S. O. Chan, T. S. A. Hof, K. L. Tan, and L. Ying-Phooi. Inorganica Chim. Acta, 1991, 184, 1, 23–26. DOI: https://doi.org/10.1016/S0020-1693(00)83040-6
D. H. Yang, Q. J. Xue, X. S. Zhang, H. Q. Wang, W. L. Lin, and X. J. Ding. Wear, 1994, 173, 1–2, 129–135. DOI: https://doi.org/10.1016/0043-1648(94)90265-8
L. S. Dake, D. R. Baer, D. M. Friedrich, L. S. Dake, D. R. Baer, and D. M. Friedrich. J. Vac. Sci. Technol. A, 2014, 1634, 1989. DOI: https://doi.org/10.1116/1.576062
P. Lo, W. Tsai, J. Lee, and M. Hung. Surf. Coatings Technol., 1994, 67, 1, 27–34. DOI: https://doi.org/10.1016/S0257-8972(05)80023-4
I. M. Watson, J. A. Connor, R. Whyman, and T. Heath. Thin Solid Films, 1991, 201, 337–349. DOI: https://doi.org/10.1016/0040-6090(91)90122-E
J. P. Delville, E. Hugonnot, C. Labrugère, T. Cohen-Bouhacina, and M. H. Delville. J. Phys. Chem. C, 2010, 114, 46, 19782–19791. DOI: https://doi.org/10.1021/jp109283v
Y. Liu, H. Lin, and C. Mou. Langmuir, 2004, 20, 8, 3231–3239. DOI: https://doi.org/10.1021/la0358421
K. H. Nam and L. L. Tavlarides, Chem. Mater., 2005, 17, 6, 1597–1604. DOI: https://doi.org/10.1021/cm048269v
D. Das, M. K. Sureshkumar, K. Radhakrishnan, J. Nuwar, and C. G. S. Pillai. J. Radioanal. Nucl. Chem., 2011, 289, 1, 275. DOI: https://doi.org/10.1007/s10967-011-1074-2
Y.-S. Yun, D. Park, J. M. Park, and B. Volesky. Environ. Sci. Technol., 2001, 35, 21, 4353–4358. DOI: https://doi.org/10.1021/es010866k
T. Cordero, J. Rodriguez-Mirasol, N. Tancredi, J. Piriz, G. Vivo, and J. J. Rodriguez. Ind. Eng. Chem. Res., 2002, 41, 24, 6042–6048. DOI: https://doi.org/10.1021/ie020210f
M. A.S.D. Barros, E.A. Silva, P.A. Arroyo, C.R.G. Tavares, R.M. Schneider, M. Suszek, E.F. Sousa-Aguiar. Chem Eng Sci.. 2004. 59 5959 – 5966. DOI: https://doi.org/10.1016/j.ces.2004.07.040
Y-G. Chen, Yong He, Wei-Min Ye, Ling-Yan Jia. J. Ind Eng Chem. 26 (2015) 335–339. DOI: https://doi.org/10.1016/j.jiec.2014.12.006
Dybowski, C.; Bai, S. Anal. Chem. 2000, 72, 1R-7R DOI: https://doi.org/10.1021/a1000002r
Eichele, K.; Ossenkamp, G. C.; Wasylishen, R.E.; Cameron, T. S. Inorg. Chem. 1999, 38, 639-651. DOI: https://doi.org/10.1021/ic9806232
Olivieri, A.C. Solid State Nuclear Magnetic Resonance (1997), 10, 19-24. DOI: https://doi.org/10.1016/S0926-2040(97)00023-4
Kempermann, H.; Bain, A. D.; Dumont R.S. J. Chem. Phys. (2002), 116, 2464-2471. DOI: https://doi.org/10.1063/1.1433003
Bemi, L.; Clark, H. C.; Davies, J. A.; Fyfe, C. A.; Wasylishen, R. E. J. Am. Chem. Soc. 1982, 104, 438-445. DOI: https://doi.org/10.1021/ja00366a011

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









