Synthesis, biological evaluation and docking study of possible antifungal compounds with a coumarin-containing triazole side chain
DOI:
https://doi.org/10.29356/jmcs.v63i2.751Keywords:
Docking, Coumarin, CYP51, Antifungal activity, Candida yeastsAbstract
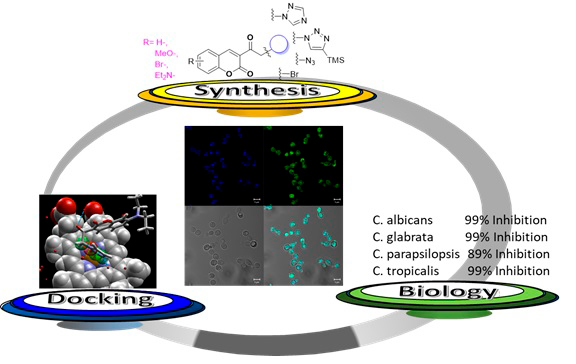
Abstract. Due to increasing drug resistance by Candida species, especially in hospitals, the search for new antifungal agents has intensified. The incorporation of the coumarin scaffold into several nitrogen-containing heterocyclic moieties reportedly increases antimicrobial efficiency. The aim of this study was to design and synthesize a series of simple coumarin-linked triazole derivatives and test their possible antifungal activity against four Candida species. Docking simulations were conducted to explore the binding properties of the test compounds and compare them to reported data on fluconazole, the reference drug. Starting from 3-acetylcoumarins, coumarins 2a-d, 3a-c and 4a-d were obtained in high yields. The concentration of each compound needed to inhibit the Candida species was determined by serial dilution. An inhibition of 62% of C. albicans was produced by 2b (300 µg/ml), 87% of C. tropicalis by 3a (100 µg/ml), 89% of C. parapsilosis by 3a (500 µg/ml), and 87% of C. glabrata by 4a (300 µg/ml). The values of antifungal activity were similar for the coumarin derivatives and fluconazole, the latter of which induced 90% inhibition of the four yeasts at 500 µg/ml. According to the docking simulations, the interactions at the active site of the lanosterol 1,4-demethylase enzyme (CYP51) are similar for the test compounds and fluconazole. The subcellular location of the derivatives was identified as the mitochondrion. These coumarins are characterized by structural simplicity, with the simplest structures showing better antifungal activity than fluconazole. Further research is needed to isolate CYP51 and directly test its inhibition by coumarin derivatives.
Resumen. Una serie de moléculas de cumarina-triazol se sintetizaron y evaluaron contra diferentes especies de Candida. Las cumarinas 2a-d, 3a-c y 4a-d se obtuvieron utilizando como material de partida las 3-acetilcumarinas en altos rendimientos. La concentración necesaria de las moléculas para mostrar actividad antifúngica contra las cuatro especies de Candida se determinó mediante un método de diluciones seriadas. Se reporta un 62% de inhibición de C. albicans usando 2b (300 µg/ml), 87% de inhibición contra C. parapsilosis por 3a (500 µg/ml), y un 87% de inhibición a C. glabrata por 4a (300 µg/ml). El efecto de las cumarinas es comparado con el fármaco de referencia fluconazol, que induce un 90% de inhibición en todas las cepas usando 500 µg/ml. Los resultados del estudio Docking muestran que las interacciones de todas las moléculas en el sitio activo de la enzima CYP51 son similares a las interacciones presentadas por el fluconazol. Finalmente, tomando ventaja de las propiedades fluorescentes de las cumarinas, la localización subcelular y penetración de los compuestos, fue localizada en las mitocondrias. Las cumarinas reportadas, además de presentar sencillez estructural, también presentan valores de inhibición de las cepas comparables, y en los casos mencionados, mejores que el fármaco de referencia.
Downloads
References
Wu, L.; Wang, X.; Xu, W.; Xu, F. F. and R. Current Medicinal Chemistry. 2009, pp 4236–4260.
Arshad, A.; Osman, H.; Bagley, M. C.; Lam, C. K.; Mohamad, S.; Zahariluddin, A. S. M. Eur. J. Med. Chem. 2011.
Renuka, N.; Ajay Kumar, K. Bioorganic Med. Chem. Lett. 2013, 23 (23), 6406–6409.
Bansal, Y.; Sethi, P.; Bansal, G. Med. Chem. Res. 2013, 22 (7), 3049–3060.
Keri, R. S.; Sasidhar, B. S.; Nagaraja, B. M.; Santos, M. A. Eur. J. Med. Chem. 2015, 100, 257–269.
Dalla Via, L.; Gia, O.; Marciani Magno, S.; Santana, L.; Teijeira, M.; Uriarte, E. J. Med. Chem. 1999, 42 (21), 4405–4413.
Kumar, R.; Saha, A.; Saha, D. Fitoterapia 2012, 83 (1), 230–233.
Hwu, J. R.; Singha, R.; Hong, S. C.; Chang, Y. H.; Das, A. R.; Vliegen, I.; De Clercq, E.; Neyts, J. Antiviral Res. 2008, 77 (2), 157–162.
Gomez-Outes, A.; Suarez-Gea, M. L.; Calvo-Rojas, G.; Lecumberri, R.; Rocha, E.; Pozo-Hernandez, C.; Vargas-Castrillon, A. I. T.-F. and E. Current Drug Discovery Technologies. 2012, pp 83–104.
Zhang, R.-R.; Liu, J.; Zhang, Y.; Hou, M.-Q.; Zhang, M.-Z.; Zhou, F.; Zhang, W.-H. Eur. J. Med. Chem. 2016, 116, 76–83.
Welsch, M. E.; Snyder, S. A.; Stockwell, B. R. Curr. Opin. Chem. Biol. 2010, 14 (3), 347–361.
Carpinella, M. C.; Ferrayoli, C.; Palacios, S. M. J. Agric. Food Chem. 2005, 53, 2922–2927.
Ji, Q.; Ge, Z.; Ge, Z.; Chen, K.; Wu, H.; Liu, X.; Huang, Y.; Yuan, L.; Yang, X.; Liao, F. Eur. J. Med. Chem. 2016, 108, 166–176.
Pfaller, M. A.; Diekema, D. J. Clin. Microbiol. Rev. 2007, 20 (1), 133–163.
Thati, B.; Noble, A.; Rowan, R.; Creaven, B. S.; Walsh, M.; McCann, M.; Egan, D.; Kavanagh, K. Toxicol. Vitr. 2007, 21 (5), 801–808.
Bowyer, P.; Moore, C. B.; Rautemaa, R.; Denning, D. W.; Richardson, M. D. Curr. Infect. Dis. Rep. 2011, 13 (6), 485.
Jeu, L.; Piacenti, F. J.; Lyakhovetskiy, A. G.; Fung, H. B. Clin. Ther. 2003, 25 (5), 1321–1381.
Pfaller, M. A.; Messer, S. A.; Hollis, R. J.; Jones, R. N. Antimicrob. Agents Chemother. 2001, 45 (10), 2862–2864.
Shaikh, M. H.; Subhedar, D. D.; Khan, F. A. K.; Sangshetti, J. N.; Shingate, B. B. Chinese Chem. Lett. 2015, 27 (2), 295–301.
Lepesheva, G. I.; Zaitseva, N. G.; Nes, W. D.; Zhou, W.; Arase, M.; Liu, J.; Hill, G. C.; Waterman, J. Biol. Chem. 2006, 281 (6), 3577–3585.
Verras, A.; Alian, A.; Ortiz De Montellano, P. R. Protein Eng. Des. Sel. 2006, 19 (11), 491–496.
W., R. V.; F., S. T.; Luciana, T.; V., S. M.; C., C. H.; R., R. C.; A., A. P. Fundam. Clin. Pharmacol. 2016, 31 (1), 37–53.
Gouda, M. A.; Berghot, M. A.; Baz, E. A.; Hamama, W. S. Med. Chem. Res. 2012, 21 (7), 1062–1070.
Raghu, M.; Nagaraj, A.; Reddy, C. S. J. Heterocycl. Chem. 2009, 46 (2), 261–267.
Musa, M. A.; Cooperwood, J. S.; Khan, M. O. F. Pharmacotherapy of Breast Cancer.; 2008; Vol. 15.
Muhammad Asif. Chem. Int. 2015, 1 (1), 1–11.
García, S.; Vázquez, J. L.; Rentería, M.; Aguilar-Garduño, I. G.; Delgado, F.; Trejo-Durán, M.; García-Revilla, M. A.; Alvarado-Méndez, E.; Vázquez, M. A. Opt. Mater. (Amst). 2016, 62, 231–239.
Lee, J. C.; Bae, Y. H.; Chang, S. K. Bull. Korean Chem. Soc. 2003, 24 (4), 407–408.
Kusanur, R. A.; Kulkarni, M. V. Indian J. Chem. - Sect. B Org. Med. Chem. 2005, 44 (3), 591–594.
Kushwaha, K.; Kaushik, N.; Lata; Jain, S. C. Design and Synthesis of Novel 2H-Chromen-2-One Derivatives Bearing 1,2,3-Triazole Moiety as Lead Antimicrobials; 2014; Vol. 24.
Thomsen, R.; Christensen, M. H. J. Med. Chem. 2006, 49 (11), 3315–3321.
Ji, H.; Zhang, W.; Zhang, M.; Kudo, M.; Aoyama, Y.; Yoshida, Y.; Sheng, C.; Song, Y.; Yang, S.; Zhou, Y.; et al. J. Med. Chem. 2003, 46 (4), 474–485.
Villaseñor-Granados, T.; García, S.; Vazquez, M. A.; Robles, J. Theor. Chem. Acc. 2016, 135 (9), 210.
Rentería Gómez, M.; López Vallejo, F. I.; Alcaraz Contreras, Y.; Flores Martínez, A.; Martínez Rosales, J. M.; Vázquez Guevara, M. Acta Univ. 2011, 21 (Regular), 74–81.
Mense, S. M.; Zhang, L. Cell Res. 2006, 16, 681.
Shingu-Vazquez, M.; Traven, A. Eukaryot. Cell 2011, 10 (11), 1376–1383.
Benhamou, R. I.; Bibi, M.; Steinbuch, K. B.; Engel, H.; Levin, M.; Roichman, Y.; Berman, J.; Fridman, M. ACS Chem. Biol. 2017, 12 (7), 1769–1777.
Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D. W.; Azeredo, J. FEMS Microbiol Rev 2012, 36, 288–305.
Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









