Effect of Electrolyte Concentration in Process Water on Flocculation
DOI:
https://doi.org/10.29356/jmcs.v64i1.720Keywords:
Coagulation, Flocculation, Electrolyte solutions, Intrinsic viscosityAbstract
Abstract. The effect of electrolyte concentration and potential determining ions on the coagulation and flocculation of illite, dolomite, and illite-dolomite mixture suspensions was investigated. Electrokinetic measurements, settling rate tests, and viscosity measurements were performed to examine the stability of these mineral suspensions and to characterize flocculants under various physico-chemical conditions. Two flocculants: A-100 anionic polyacrylamide (PAM) and polyethylene oxide (PEO) were employed.
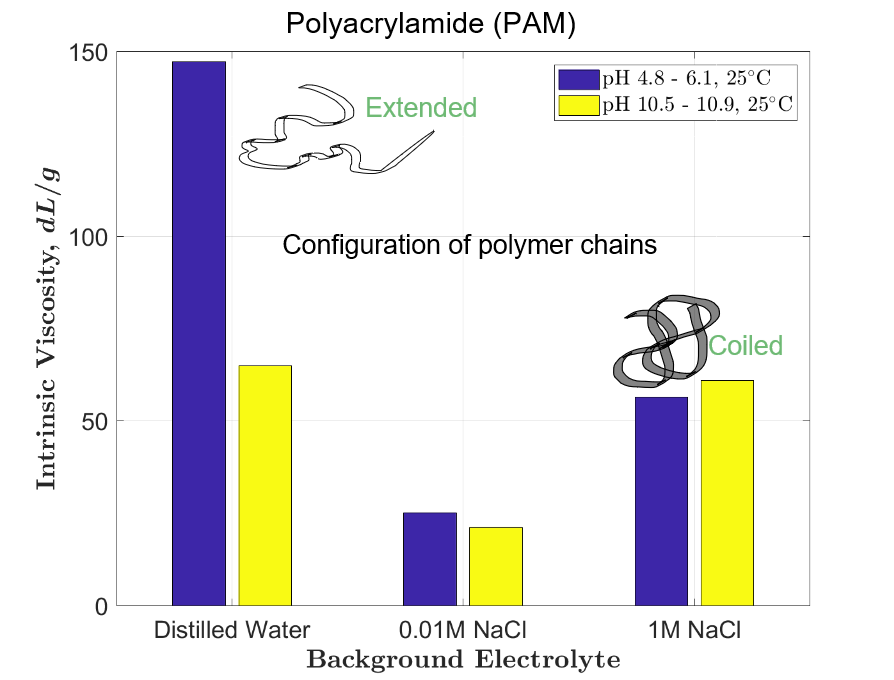
The tests revealed that polyethylene oxide does not flocculate dolomite under any tested conditions. Viscosity results corroborated that the conformation of PAM macro- molecules in water is very sensitive to electrolyte concentration; on the other hand, the conformational state of PEO macromolecules is not affected by ionic strength. The intrinsic viscosity measurements suggest that the unattainable flocculation of dolomite suspensions with PEO must result from poor adsorption of this flocculant onto dolomite particles. In both tested cases, with PAM and PEO, the relationship between coagulation and flocculation was not confirmed.
Resumen. Se investigó el efecto de la concentración electrolítica y iones determinantes de potencial en la coagulación y floculación de suspensiones de ilita, dolomita y mezcla de ilita-dolomita. Mediciones electrocinéticas, ensayos de velocidad de sedimentación, y mediciones de viscosidad fueron realizadas para examinar la estabilidad de estas suspensiones minerales y caracterizar floculantes bajo varias condiciones fisicoquímicas. Se empleó dos floculantes: A-100 poliacrilamida aniónica (PAM) y óxido de polietileno (PEO).
Los experimentos revelaron que el óxido de polietileno no flocula la dolomita bajo ninguna condición empleada. Los resultados de viscosidad corroboraron que la conformación de las macromoléculas PAM en agua es sensible a la concentración electrolítica; por otro lado, el estado conformacional de las macromoléculas de PEO no se ve afectado por la fuerza iónica. Las mediciones de viscosidad intrínseca sugieren que la floculación inalcanzable de las suspensiones de dolomita con PEO debe ser resultado de una mala adsorción de este floculante en las partículas de dolomita. En ambos casos probados, con PAM y PEO, no se confirmó la relación entre coagulación y floculación.
Downloads
References
Laskowski, J. S., in: Coal Flotation and Fine Coal Utilization, Elsevier Science B.V., 2001.
Michaels, A. S. Ind. Engnineering Chem. Chem. 1954, 46, 1485–1490. DOI: https://doi.org/10.1021/ie50538a053.
Hogg, R., Polymer Adsorption and Flocculation. Polymers in Mineral Processing - Proc. of the Third UBC-McGill International Symposium on Fundamentals of Mineral Processing; Laskowski, J. S., Ed.; Metallurgical Society of CIM: Quebec City, 1999; pp 3–18.
Attia, Y. A. Flocculation. In Colloid chemistry in mineral processing; Laskowski, J. S., Ralston, J., Eds.; Elsevier Science Publishers B.V., 1992; pp 277–308.
Arinaitwe, E.; Pawlik, M. Int. J. Miner. Process. 2009, 91, 50–54. DOI: https://doi.org/10.1016/j.minpro.2008.12.002.
Mpofu, P.; Addai-mensah, J.; Ralston, J. Int. J. Miner. Process. 2003, 71, 247–268. DOI: https://doi.org/10.1016/S0301-7516(03)00062-0.
Nasser, M. S.; James, A. E. Sep. Purif. Technol. 2006, 52, 241–252. DOI: https://doi.org/10.1016/j.seppur.2006.04.005.
Addai-Mensah, J. Powder Technol. 2007, 179, 73–78. DOI: https://doi.org/10.1016/j.powtec.2006.11.008.
Kitchener, J. A. Br. Polym. J. 1972, 4, 217–229. DOI: https://doi.org/10.1002/pi.4980040307.
Hogg, R. Coal Preparation Wastewater and Fine Refuse Treatment. In Fine Coal Processing; Noyes Publications, 1987; 269–293.
Onen, V.; Gocer, M. Part. Sci. Technol. 2018, 1–8. DOI: https://doi.org/10.1080/02726351.2018.1454993.
Rubio, J. Colloids and Surfaces. 1981, 3, 79–95. DOI: https://doi.org/10.1016/0166-6622(81)80035-2.
Laskowski, J.; Castro, S. Int. J. Miner. Process. 2015, 144, 50–55. DOI: https://doi.org/10.1016/j.minpro.2015.09.017.
Ferrera, V.; Arinaitwe, E.; Pawlik, M. A Role of Flocculant Conformation in the Flocculation Process. In Proceedings of the 7th UBC-McGill-UA Symposium on Fundamentals of Mineral Processing; Gomez, C. O., Nesset, J. E., Rao, S. R., Eds.; Montreal, 2009; 397–408.
Friend, J. P.; Kitchener, J. A. Chem. Eng. Sci. 1973, 28, 1071–1080. DOI: https://doi.org/10.1016/0009-2509(73)80010-7.
Huang, P.; Laskowski, J. S.; Zeng, H.; Lu, Q. Use of Flocculants in High Ionic Strength Process Waters. In 9th UBC-McGill-UA Int. Symposium; 2013.
Yu, K. Lab Report: Flocculation with the Use of Polyacrylamide as a Flocculant in NaCl Solutions; Vancouver, 2015.
Arinaitwe, E. Characterization of Industrial Flocculants Through Intrinsic Viscosity Measurements, MASc Thesis. The University of British Columbia, Canada, 2008. DOI: https://doi.org/10.14288/1.0066521.
Kulicke, W.-M.; Kniewske, R.; Klein, J. Prog. Polym. Sci. 1982, 8, 373–468.
Kulicke, W.-M.; Clasen, C. Viscosimetry of Polymers and Polyelectrolytes; Springer, Berlin, Heidelberg, 2004. DOI: https://doi.org/10.1002/pi.1722.
Lide, D. R. CRC Handbook of Chemistry and Physics, 88th ed.; CRC Press, 2007. DOI: https://doi.org/10.4324/9781410610348.
Fedors, R. F. Polymer (Guildf). 1979, 20, 225–228. DO: https://doi.org/10.1016/0032-3861(79)90226-X.
Moudgil, B. M.; Mathur, S.; Behl, S. Miner. Metall. Process. 1995, 24–27.
Somasundaran, P. J. Colloid Interface Sci. 1967, 24, 433–440. DOI: https://doi.org/10.1016/0021-9797(67)90241-X.
Marouf, R.; Marouf-Khelifa, K.; Schott, J.; Khelifa, A. Microporous Mesoporous Mater. 2009, 122, 99–104. DOI: https://doi.org/10.1016/j.micromeso.2009.02.021.
Gence, N.; Ozbay, N. Appl. Surf. Sci. 2006, 252, 8057–8061. DOI: https://doi.org/10.1016/j.apsusc.2005.10.015.
Pokrovsky, O. S.; Schott, J.; Thomas, F. Geochim. Cosmochim. Acta. 1999, 63, 3133–3143. DOI: https://doi.org/10.1016/S0016-7037(99)00240-9.
Liu, Y.; Liu, Q. Miner. Eng. 2004, 17, 865–878. DOI: https://doi.org/10.1016/j.mineng.2004.03.007.
Ding, K.; Laskowski, J. S. Can. Metall. Q. 2006, 45, 199–206. DOI: https://doi.org/10.1179/cmq.2006.45.2.199.
Kasha, A.; Al-Hashim, H.; Abdallah, W.; Taherian, R.; Sauerer, B. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 482, 290–299. DOI: https://doi.org/10.1016/j.colsurfa.2015.05.043.
Johnson, S. B.; Franks, G. V.; Scales, P. J.; Boger, D. V.; Healy, T. W. Int. J. Miner. Process. 2000, 58, 267–304. DOI: https://doi.org/10.1016/S0301-7516(99)00041-1.
Laskowski, J. S. CIM J. 2012, 3, 203–214.
Tombácz, E.; Szekeres, M. Appl. Clay Sci. 2006, 34, 105–124. DOI: https://doi.org/10.1016/j.clay.2006.05.009.
Hogg, R. Int. J. Miner. Process. 2000, 58, 223–236. DOI: https://doi.org/10.1016/S0301-7516(99)00023-X.
Scheiner, B. J.; Wilemon, G. M. Applied Flocculation Efficiency: A Comparison of Polyethylene Oxide and Polyacrylamides. In Flocculation in Biotechnology and Separation Systems; Attia, Y. A., Ed.; Elsevier Science Publishers B.V.: Amsterdam, 1987; Vol. 4, 175–185.
Sworska, A.; Laskowski, J. S.; Cymerman, G. Int. J. Miner. Process. 2000, 60, 143–152. DOI: https://doi.org/10.1016/S0301-7516(00)00012-0.
Rubio, J.; Kitchener, J. A. J. Colloid Interface Sci. 1976, 57, 132–142. DOI: https://doi.org/10.1016/0021-9797(76)90182-X.
Moudgil, B. M.; Chanchani, R. Trans. Am. Inst. Mining, Metall. Pet. Eng. 1985, 278 (March 1983), 13–19.
Moudgil, B. M.; Mathur, S.; Behl, S. Miner. Metall. Process. 1995, 12, 219–224.
Mathur, S.; Moudgil, B. Miner. Metall. Process. 1998, 15, 24–28.
Ramirez, A.; Rojas, A.; Gutierrez, L.; Laskowski, J. S. Miner. Eng. 2018, 125 (November 2017), 10–14. DOI: https://doi.org/10.1016/j.mineng.2018.05.008.
Andreola, F.; Castellini, E.; Ferreira, J. M. F.; Olhero, S.; Romagnoli, M. Appl. Clay Sci. 2006, 31, 56–64. DOI: https://doi.org/10.1016/j.clay.2005.08.004.
Pawlik, M.; Laskowski, J. S.; Ansari, A. J. Colloid Interface Sci. 2003, 260, 251–258. DOI: https://doi.org/10.1016/S0021-9797(02)00225-4.
Gochin, R. J.; Leklll, M.; Shergold, H. L. Coal Prep. 1985, 2 (May 2012), 19–33. DOI: https://doi.org/10.1080/07349348508905150.
Mpofu, P.; Addai-Mensah, J.; Ralston, J. J. Colloid Interface Sci. 2004, 271, 145–156. DOI: https://doi.org/10.1016/j.jcis.2003.09.042.
Huggins, M. L. J. Am. Chem. Soc. 1942, 64, 2716–2718. DOI: https://doi.org/10.1021/ja01263a056.
Sakai, T. J. Polym. Sci. 1968, 6, 1535–1549.
Arinaitwe, E.; Pawlik, M. Int. J. Miner. Process. 2013, 124, 50–57.
Napper, D. H. J. Colloid Interface Sci. 1977, 58, 390–407. DOI: https://doi.org/10.1016/0021-9797(77)90150-3.
Ma, X.; Pawlik, M. Can. Metall. Q. 2007, 46, 321–327. DOI: https://doi.org/10.1179/cmq.2007.46.3.321.
Rao, M. V. S. Polymer (Guildf). 1993, 34, 592–596. DOI: https://doi.org/10.1016/0032-3861(93)90555-O.
Ghimici, L.; Popescu, F. Eur. Polym. J. 1998, 34, 13–16. DOI: https://doi.org/10.1016/S0014-3057(97)00072-4.
Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









