What comparisons of natural and chimeric contacts reveal about inhibition of human cathepsins K, L and S by their prosegments
DOI:
https://doi.org/10.29356/jmcs.v63i1.684Keywords:
human cathepsins, cathepsin inhibition, proteinase chimeric complexes, protease prosegments, cysteine proteasesAbstract
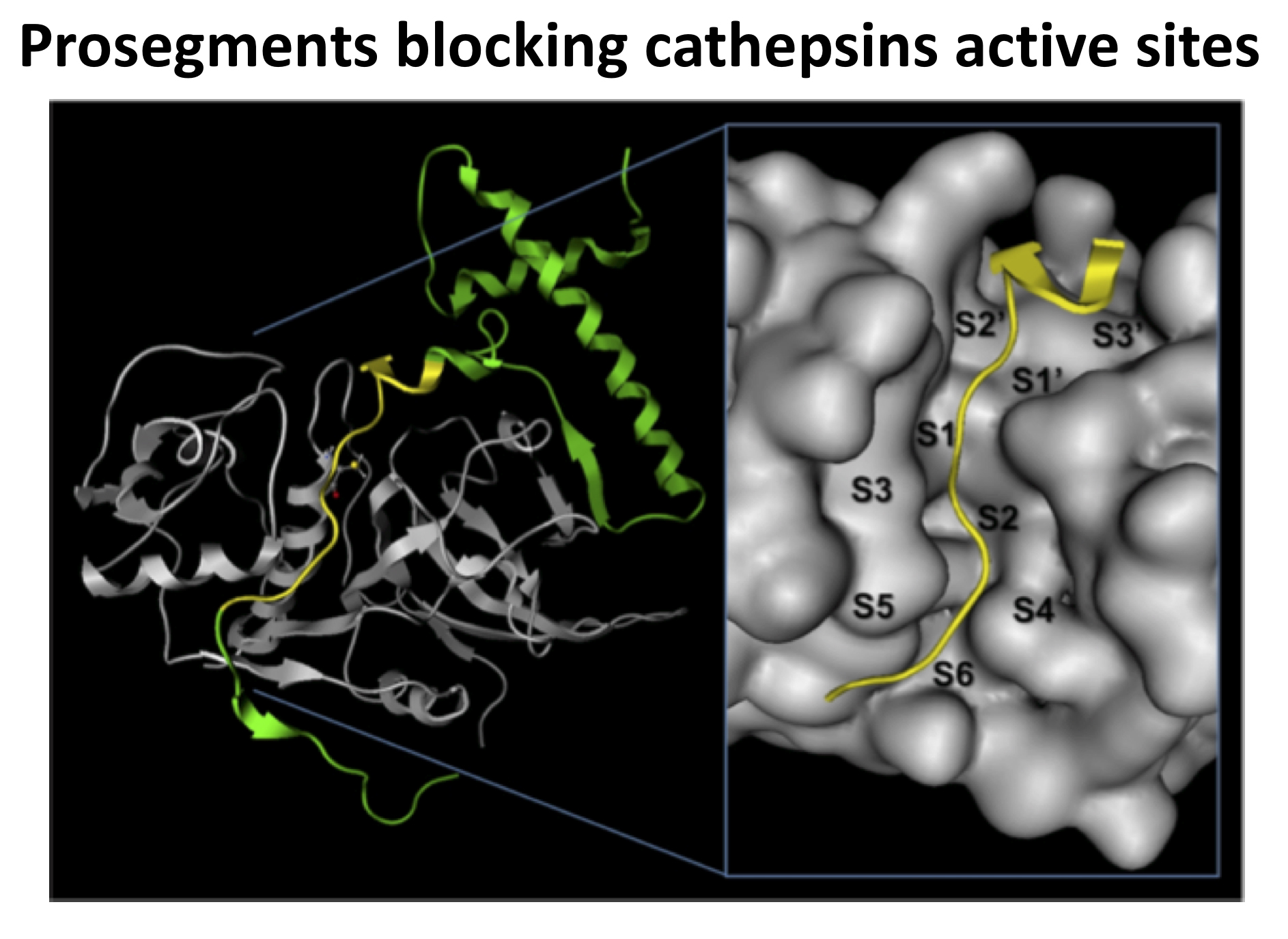
Human cathepsins K, L, and S, which are involved in the development of several serious diseases, are strongly inhibited by their related prosegments, to which they are covalently bound or simply forming complexes. In this work, three-dimensional structures of the three natural complexes of these enzymes with their related proregions were constructed, as well as six chimeric complexes of the same three prosegments with their non-cognate enzymes. We made a comparative study of the contacts in all nine structures throughout their active sites. The analysis was performed looking for a structural parameter that could agree with the values of the inhibition constants reported experimentally for each of the nine complexes. We found that this correlating parameter was the difference of the electrostatic energy (involving hydrogen bonds and ion pairs) at the binding interface of a 13-amino acid fragment of the prosegments. We used the results of this work, on the one hand, to identify the key residues involved in the electrostatic intermolecular recognition in each studied complex and, on the other, to explain some results achieved by different research groups on the inhibition of the same enzymes analyzed here. It was found that the natural cathepsin L complex showed a higher number of electrostatic interactions, some of them interconnected, when compared to the other two natural complexes. In addition, the chimeric contacts revealed binding sites that could be used to achieve a more potent inhibition of these cathepsins, avoiding cross-interactions.
Downloads
References
Schick, C.; Pemberton, P.A.; Shi, G.P.; Kamachi, Y.; Cataltepe, S.; Bartuski, A.J.; Gornstein, E.R.; Bromme, D.; Chapman, H.A.; Silverman, G.A. Biochem. 1998, 37, 5258-5266. DOI: https://doi.org/10.1021/bi972521d
Palermo, C.; Joyce. J.A. Trends Pharmacol Sci. 2007, 29, 22-28. DOI: https://doi.org/10.1016/j.tips.2007.10.011
Schornberg, K.; Matsuyama, S.; Kabsch, K.; Delos, S.; Bouton, A.; White, J. J. Virol. 2006, 80, 4174-4178. DOI: https://doi.org/10.1128/JVI.80.8.4174-4178.2006
Lankelma, J.M.; Voorend, D.M.; Barwari, T.; Koetsveld, J.; Van der Spek, A.H.; De Porto, A.P.N.A.; Van Rooijen, G.; Van Noorden, C.J.F. Life Sciences. 2010, 86, 225–233. DOI: https://doi.org/10.1016/j.lfs.2009.11.016
Bromme, D.; Klaus, J.L.; Okamoto, K.; Rasnick, D.; Palmer, J.T. Biochem. J. 1996, 315, 85-89. DOI: https://doi.org/10.1042/bj3150085
Cywin, C.L.; Firestone, R.A.; McNeil, D.W.; Grygon, C.A.; Crane, K.M.; White, D.M.; Kinkade, P.R.; Hopkins, J.L.; Davidson, W.; Labadia, M.E.; Wildeson, J.; Morelock, M.M.; Peterson, J.D.; Raymond, E.L.; Brownand, M.L.; Spero, D.M. Bioorg. Med. Chem. 2003, 11, 733-740. DOI: https://doi.org/10.1016/S0968-0896(02)00468-6
Gauthier, J.Y.; Chauret, N.; Cromlish, W.; Desmarais, S.; Duong, L.T.; Falgueyret, J.P.; Kimmel, D.B.; Lamontagne, S.; Le´ger, S., Le Riche, T.; Sing, C.; Masse´, L.F.; McKay, D.J.; Nicoll-Griffith, D.A.; Oballa, R.M.; Palmer, J.T.; Percival, M.D.; Riendeau, D.; Robichaud, J.; Rodan, G.A.; Rodan, S.B., Seto, C.; The´rien, M.; Truong, V.L.; Venuti, M.C.; Wesolowski, G.; Young, R.N.; Zambonia, R.; Black, W.C. Bioorg. Med. Chem. Lett. 2008, 18, 923-928. DOI: https://doi.org/10.1016/j.bmcl.2007.12.047
Stoch, S.A.; Wagner, J.A.Clin. Pharmacol. Ther. 2008, 83, 172-176. DOI: https://doi.org/10.1038/sj.clpt.6100450
Löser, R.; Pietzsch, J. Cysteine cathepsins. Front Chem. 2015, 23, 3-37. DOI: https://doi.org/10.3389/fchem.2015.00037
Kramer, L.; Turk, D.; Turk, B. Trends Pharmacol Sci. 2017, 38, 873-889. DOI: https://doi.org/10.1016/j.tips.2017.06.003
Coulombe, R.; Grochulski, P.; Sivaraman, J.; Ménard, R.; Morton, J.S. Cygler, M. EMBO J. 1996, 15, 5492-5503. DOI: https://doi.org/10.1002/j.1460-2075.1996.tb00934.x
Maubach, G.; Schilling, K.; Rommerskirch, W.; Wenz, I.; Schultz, J.E.; Weber, E.; Wiederanders, B. Eur. J. Biochem. 1997, 250, 745-750. DOI: https://doi.org/10.1111/j.1432-1033.1997.00745.x
Billington, C.J.; Mason, P.; Magny, M.C.; Mort, J.S. Biochem. Biophys. Res. Commun. 2000, 276, 924-929. DOI: https://doi.org/10.1006/bbrc.2000.3553
Nomura, T.; Fujisawa, Y. Biochem. Biophys. Res. Commun. 1997, 230, 143-146. DOI: https://doi.org/10.1006/bbrc.1996.5905
Ishidoh, K.; Saido, T.C.; Kawashima, S.; Hirose, M.; Watanabe, S.; Sato, N.; Kominami, E. Biochem. Biophys. Res. Commun. 1998, 252, 202-207. DOI: https://doi.org/10.1006/bbrc.1998.9613
Ménard, R.; Carmona, E.; Takebe, S.; Dufour, E.; Plouffe, C.; Mason, P.; Mort, J.S. J. Biol. Chem. 1998, 273, 4478-4484. DOI: https://doi.org/10.1074/jbc.273.8.4478
Rieman, D.J.; McClung, H.A.; Dodds, R.A.; Hwang, S.M.; Holmes, M.W.; James, I.E.; Drake, F.H.; Gowen, M. Bone. 2001, 28, 282-289. DOI: https://doi.org/10.1016/S8756-3282(00)00445-2
Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. Nucleic Acids Res. 2000, 28, 235-242. DOI: https://doi.org/10.1093/nar/28.1.235
Schechter, I.; Berger, A. Biochem. Biophys. Res. Commun. 1967, 27, 157-162. DOI: https://doi.org/10.1016/S0006-291X(67)80055-X
Turk, V.; Stoka, V.; Vasiljeva, O.; Renko, M.; Sun, T.; Turk, B.; Turk, D. Biochim. Biophys. Acta. 2012, 1824, 68-88. DOI: https://doi.org/10.1016/j.bbapap.2011.10.002
Guay, J.; Falgueyret, J.P.; Ducret, A.; Percival, M.D.; Mancini, J.A. Eur. J. Biochim. 2000, 267, 6311-6318. DOI: https://doi.org/10.1046/j.1432-1327.2000.01730.x
Wiederanders, B. Acta. Biochim. Pol. 2003, 50, 691. DOI: https://doi.org/10.18388/abp.2003_3661
Ang, K.K.H.; Ratnam, J.; Gut, J.; Legac J.; Hansell, E.; Mackey, Z.B.; Skrzypczynska, K.M.; Debnath, A.; Engel, J.C.; Rosenthal, P.J.; McKerrow, J.H.; Arkin, M.R. and Renslo, A.R. PLoSNegl. Trop. Dis. 2011, 5, e1023, doi:10.1371/journal.pntd.0001023. DOI: https://doi.org/10.1371/journal.pntd.0001023
Sosic, I.; Mirkovic, B.; Arenz, K.; Stefane, B.; Kos, J. and Gobec, S. J. Med. Chem. 2012, 56, 521-533. DOI: https://doi.org/10.1021/jm301544x
Molecular Operating Environment (MOE), 2013.08; Chemical Computing Group ULC, 1010 Sherbrooke St. West, Suite #910, Montreal, QC, Canada, H3A 2R7, 2018.
Gasteiger, J.; Marsili, M. Tetrahedron. 1980, 36, 3219-3228. DOI: https://doi.org/10.1016/0040-4020(80)80168-2
Londe, J.M.L.; Zhao, B.; Janson, C.A.; D'Alessio, K.J.; McQueney, M.S.; Orsini, M.J.; Debouck, C.M.; Smith, W.W. Biochem. 1999, 19. 862-869. DOI: https://doi.org/10.1021/bi9822271
Cygler, M.; Coulombe, R. Crystal structure of procathepsin L doi: 102210/pdb1CS8/pdb.
Kaulmann, G.; Palm, G.J.; Schilling, K.; Hilgenfeld, R.; Wiederanders, B. Protein Sci. 2006, 15; 2619-2629. DOI: https://doi.org/10.1110/ps.062401806
Nucleic Acids Research, Volume 45, Issue D1, 4 January 2017, Pages D158–D169,https://doi.org/10.1093/nar/gkw1099.
Inaoka, T.; Bilbe, G.; Ishibashi, O.; Tezuka, K.; Kumegawa, M.; Kokubo, T. Biochem. Biophys. Res. Commun. 1995, 206, 89-96. DOI: https://doi.org/10.1006/bbrc.1995.1013
Gal, S.; Gottesman, M.M. Biochem. J. 1988, 253, 303-306. DOI: https://doi.org/10.1042/bj2530303
Shi, G.P. Munger, J.S.; Meara, J.P.; Rich, D.H.; Chapman, H.A. J. Biol. Chem. 1992, 267, 7258-7262. DOI: https://doi.org/10.1016/S0021-9258(18)42513-6
Söding, J.; Biegert, A.; Lupas, A.N. Nucleic Acids Res. 2005, 33, 244-248. DOI: https://doi.org/10.1093/nar/gki408
Eisenberg, D.; Lüthy, R.; Bowie, J.U. Methods Enzymol. 1997, 277, 396-404. DOI: https://doi.org/10.1016/S0076-6879(97)77022-8
Laskowski,R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. J. Appl. Cryst. 1993, 26, 283-291. DOI: https://doi.org/10.1107/S0021889892009944
Laskowski, R. A.Nucleic Acids Res. 2009, 37, D355–D359. DOI: https://doi.org/10.1093/nar/gkn860
Li, C.S.; Deschenes, D.; Desmarais, S.; Falgueyret, J.P.; Gauthier, J.Y.; Kimmel, D.B.; Léger, S.; Massé, F.; McGrath, M.E.; McKay, D.J.; Percival, M.D.; Riendeau, D.; Rodan, S.B.; Thérien, M.; Truong, V.L.; Wesolowski, G.; Zamboni, R.; Black, W.C. Bioorg. Med. Chem. Lett. 2006, 16, 1985-1989. DOI: https://doi.org/10.1016/j.bmcl.2005.12.071
Chowdhury, S.F.; Sivaraman, J.; Wang, J.; Devanathan, G.; Lachance, P.; Qi, H.; Ménard, R.; Lefebvre, J.; Konishi, Y.; Cygler, M.; Sulea, T.; Purisima, E.O. J. Med. Chem. 2002, 45, 5321-5329. DOI: https://doi.org/10.1021/jm020238t
Wiener, D.K.; Lee-Dutra, A.; Bembenek, S.; Nguyen, S.; Thurmond, R.L.; Sun, S.; Karlsson, L.; Grice, C.A.; Jones, T.K.; Edwards, J.P. Bioorg. Med. Chem. Lett. 2010, 20, 2379-2382. DOI: https://doi.org/10.1016/j.bmcl.2010.01.103
Baker, N.A.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Proc. Natl. Acad. Sci. 2001, 98, 10037-10041. DOI: https://doi.org/10.1073/pnas.181342398
Carmona, E.; Dufour, E.; Plouffe, C.; Takebe, S.; Mason, P.; Mort, J.S.; Ménard, R. Biochem. 1996, 35,8149-8157. DOI: https://doi.org/10.1021/bi952736s
Cappetta, M.; Roth, I.; Díaz, A.; Tort, J.; Roche, L. Biol. Chem. 2002, 383, 1215-122. DOI: https://doi.org/10.1515/BC.2002.134

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









