Synthesis, characterization and cytotoxic activity of tioconazole coordination compounds with nickel(II), palladium(II) and platinum(II)
DOI:
https://doi.org/10.29356/jmcs.v62i4.563Keywords:
Tioconazole, coordination compounds, nickel(II), palladium(II), platinum, intermolecular interactions, cytotoxic activityAbstract
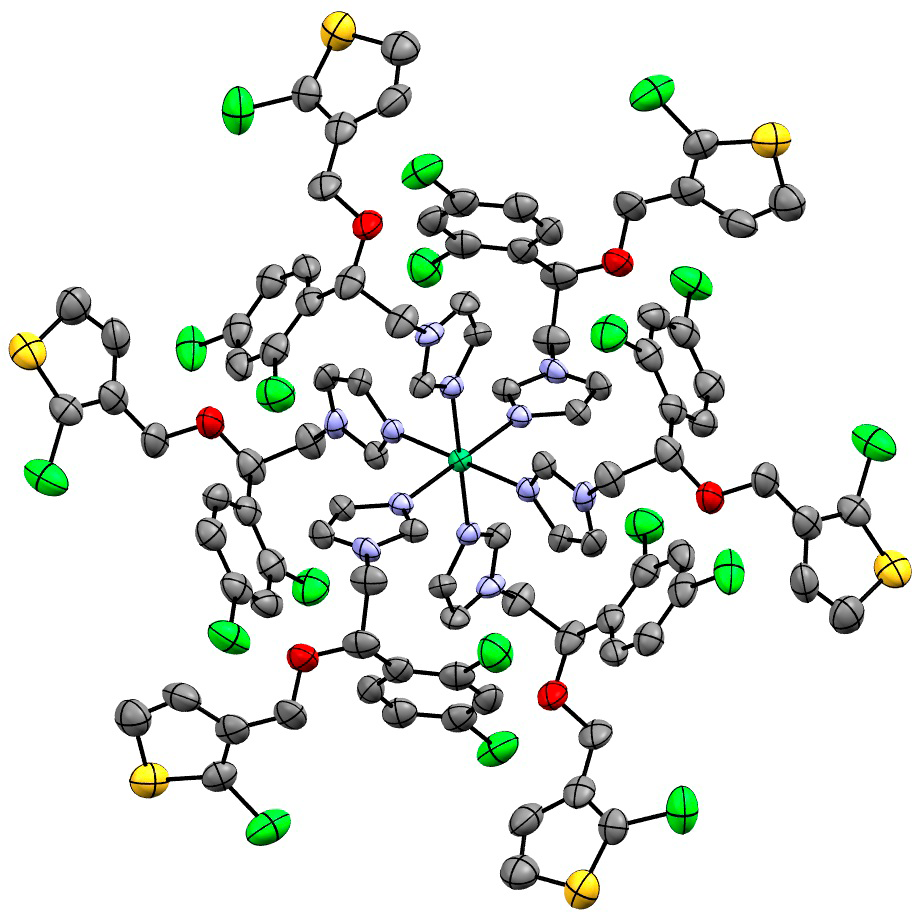
Coordination compounds of nickel(II), palladium(II) and platinum(II) with tioconazole (tcnz) were synthesized and characterized by infrared, UV-Vis-NIR, elemental analysis, molar conductivity, magnetic susceptibility, mass spectrometry, NMR spectroscopy and X- ray diffraction. Tioconazole presented a monodentate coordination mode, through the nitrogen atom of the imidazolic ring. The NiII compounds stabilized an octahedral geometry. In [Ni(tcnz)2(NO3)2].H2O the coordinated nitrate presented a bidentate coordination mode, while for the [Ni(tcnz)2(OAc)2].3H2O compound, the acetate behaves as a bridging ligand. When different molar ratios were used on the reaction synthesis, three or six ligands were coordinate to the nickel(II) atom, [Ni(tcnz)3Br2(H2O)], [Ni(tcnz)6]Cl2 and [Ni(tcnz)6]Br2. The palladium(II) and platinum(II) compounds, [Pd(tcnz)2Cl2], [Pt(tcnz)2Cl2].2H2O and [Pd(tcnz)2(OAc)2], stabilized a trans-square planar geometry. The compounds [Ni(tcnz)6]X2 give place to 3D supramolecular arrangements through hydrogen bonding (X∙∙∙H, X = Cl and Br) and p∙∙∙p stacking interactions, between the six membered rings of neighbouring molecules. The in vitro cytotoxic activity of the synthesized compounds was studied in four different human carcinoma cell lines: HCT-15 (colon), HeLa (cervix-uterine), MCF-7 (breast) and PC-3 (prostate).
Downloads
References
Castiñeiras, A.; Fernández-Hermida, N.; García-Santos, I.; Gómez-Rodríguez, L. Dalton Trans. 2012, 43, 13486-13495 DOI: https://doi.org/10.1039/c2dt31753b
Macombera, L.; Hausinger, R. P. Metallomics, 2011, 3, 1153-1162 DOI: https://doi.org/10.1039/c1mt00063b
Ragsdale, S. W. Curr. Op. Chem. Biol. 1998, 2, 208-215 DOI: https://doi.org/10.1016/S1367-5931(98)80062-8
Boer, J. L.; Mulrooney, S. B.; Hausinger, R. P. Arch. Biochem. Biophys. 2014, 544, 142- 152 DOI: https://doi.org/10.1016/j.abb.2013.09.002
Tottaa, X.; Papadopouloua, A.A.; Hatzidimitrioua, A. G.; Papadopoulos, A.; Psomas, G. J. Inorg. Biochem. 2015, 145, 79-93 DOI: https://doi.org/10.1016/j.jinorgbio.2015.01.009
Chohan, Z. H.; Arif, M.; Shafiq, Z.; Yaqub, M.; Supuran, C. T. J. Enz. Inhib. Med. Chem. 2006, 21, 95-103 DOI: https://doi.org/10.1080/14756360500456806
Williams, C. J.; Whitehouse, J. M. A., Br. Med. J. 1979, 1, 1689-1691 DOI: https://doi.org/10.1136/bmj.1.6179.1689
Langer, C.J.; Gadgeel, S. M.; Borghaei, H.; Papadimitrakopoulou, V. A.; Patnaik, A.; Powell, S. F.; Gentzler, R. D.; Martins, R.G.; Stevenson, J. P.; Jalal, S. I.; Panwalkar, A.; Yang, J. C.; Gubens, M.; Sequist, L. V.; Awad, M. M.; Fiore, J.; Ge, Y.; Raftopoulos, H.; Gandhi, L. Lancet Oncol. 2016, 11, 1497-1508 DOI: https://doi.org/10.1016/S1470-2045(16)30498-3
Williams, K.J.; Picus, J.; Trinkhaus, K.; Fournier, C. C.; Suresh, R.; James, J. S.; Tan, B. R. HPB 2010, 6, 418-426 DOI: https://doi.org/10.1111/j.1477-2574.2010.00197.x
Alcindor, T.; Beauger, N. Curr. Oncol. 2011, 1, 18-25 DOI: https://doi.org/10.3747/co.v18i1.708
Eddings, D.; Barnes, C.; Gerasimchuk, N.; Durham, P.; Domasevich, K. Inorg. Chem.
, 43, 3894-3909
Jiang, Y.; Shan, S.; Gan, T.; Zhang, X.; Lu, X.; Hu, H.; Wu, Y.; Sheng, J.; Yang, J. Biomed. Rep. 2014, 2, 893-897 DOI: https://doi.org/10.3892/br.2014.349
Xiang, K.; Hai-Hua, X.; Hai-Qin, S.; Xia-Bin, J.; Le-San, Y.; Ruo-Gu, Q. Cancer Biol. Med. 2015, 12, 362-374
Wong, E.; Giandomenico, C. M. Chem. Rev. 1999, 99, 2451-2466 DOI: https://doi.org/10.1021/cr980420v
Gao, E.; Liu, L.; Zhu, M.; Huang, Y.; Guan, F.; Gao, X.; Zhang, M.; Wang, L. Zhang, W.; Sun, Y. Inorg. Chem. 2011, 50, 4732-4741 DOI: https://doi.org/10.1021/ic102142j
Jahromi, E. Z.; Divsalar, A.; Saboury, A. A.; Khaleghizadeh, S.; Mansouri-Torshizi, H.; Kostova, I. J. Iran Chem. Soc. 2016, 13, 967-989 DOI: https://doi.org/10.1007/s13738-015-0804-8
Sharma, N. K.; Ameta, R. K.; Singh, M. Inter. J. Med. Chem. 2016, 2016, 1-10 DOI: https://doi.org/10.1155/2016/9245619
Bingchang, Z.; Haiqing, L.; Qinjuan, X.; Lirong, L.; Bing, Z. Oncotarget. 2017 8, 13620-13631 DOI: https://doi.org/10.18632/oncotarget.14620
Hartmann, J. T.; Lipp, H. Expert. Opin. Pharmacother. 2003, 6, 889-901 DOI: https://doi.org/10.1517/eoph.4.6.889.22200
Sabokrouh, A.; Vaisi-Raygani, A.; Goodarzi, M. T.; Khatami, S.; Taghizadeh-Jahed, M.; Shahabadi, N.; Lakpour, N.; Shakiba, Y. Avicenna. J. Med. Biotechnol. 2015, 7, 50-56
O'Brien, M. E.; Szczesna, A.; Karnicka, H.; Zatloukal, P.; Eisen, T.; Hartmann, W.; Kasan, P.; Longerey, B.; Lefresne, F. Annals of Oncology. 2004, 15, 921-927 DOI: https://doi.org/10.1093/annonc/mdh233
Abu-Surrah, A. S.; Abu-Safieh, K. A.; Ahmad, I. M.; Abdalla, M. Y.; Ayoub, M. T.; Qaroush, A. K.; Abu-Mahtheieh, A. M. Europ. J. Med. Chem. 2010, 45, 471-475 DOI: https://doi.org/10.1016/j.ejmech.2009.10.029
Abu-Surrah, A. S.; Al-Sa’doni, H. H.; Abdalla, M. Y. Cancer Therapy 2008, 6, 1-10
Garoufis, A.; Hadjikakou, S. K.; Hadjiliadis, N. Coord. Chem. Rev. 2009, 253, 1384- 1397 DOI: https://doi.org/10.1016/j.ccr.2008.09.011
Hernández, W.; Paz, J.; Carrasco, F.; Vaisberg, A.; Spodine, E.; Manzur, J.; Hennig, L.; Sieler, J.; Blaurock, S.; Beyer, L. Bioinorg. Chem. & Appl. 2013, 2013, 1-12 DOI: https://doi.org/10.1155/2013/524701
Ali, M. A.; Mirza, A. H.; Butcher, R.; Tarafder, M. T. H.; Keat, T. B.; Ali, A. M. J. Inorg. Biochem. 2002, 92, 141-148 DOI: https://doi.org/10.1016/S0162-0134(02)00559-7
Kljun, J.; Scott, A. J.; Rizner, T. L.; Keiser, J.; Turel, I. Organometallics. 2014 33, 1594-1601 DOI: https://doi.org/10.1021/om401096y
Singh, K.; Kumar, Y.; Puri, P.; Kumar, M.; Sharma, C. Eur. J. Med. Chem. 2012, 52, 313-321 DOI: https://doi.org/10.1016/j.ejmech.2012.02.053
Crisóstomo-Lucas, C.; García-Holley, P.; Hernández-Ortega, S.; Sánchez-Bartéz, F.; Gracia-Mora, I.; Barba-Behrens, N. Inorg. Chim. Acta. 2015, 438, 245-254 DOI: https://doi.org/10.1016/j.ica.2015.09.029
Mathews, C. J.; Smith, P. J.; Welton, T. J. Mol. Catal. A: Chem. 2004, 214, 27-32 DOI: https://doi.org/10.1016/j.molcata.2003.11.030
Sheldrick, G. M. Acta Cryst. 2008, A64, 112 DOI: https://doi.org/10.1107/S0108767307043930
Clark, R. C.; Reid, J. S. Acta Cryst. 1995, A51, 887 DOI: https://doi.org/10.1107/S0108767395007367
Hübschle, C. B.; Sheldrick, G. M.; Dittrich, B. J. Appl. Cryst. 2011, 44, 1281-1284 DOI: https://doi.org/10.1107/S0021889811043202
Rubinstein, L. V.; Shoemaker, R. H.; Paull, K. D.; Simon, R. M.; Tosini, S.; Skehan, P.; Scudiero, D. A.; Monks, A.; Boyd, M. R. J . Nat. Cancer Inst. 1990, 82, 1113-1117 DOI: https://doi.org/10.1093/jnci/82.13.1113
Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J. T.; Bokesch, H.; Kenney, S.; Boyd, M. R. J Nat. Cancer Inst. 1990, 82, 1107-1112 DOI: https://doi.org/10.1093/jnci/82.13.1107
Alley, M. C.; Scudiero, D. A.; Monks, A.; Hursey, M. L.; Czerwinski, M. J.; Fine, D. L.; Abbott, B. J.; Mayo, J. G.; Shoemaker, R. H.; Boyd, M. R. Cancer Res. 1988, 48, 589- 601
Nakamoto, K. Infrared and Raman Spectra of Inorganic Coordination Compounds, John Wiley & Sons, 1986
Bourke, J. P.; Cannon, R. D.; Grinter, G.; Jayasooriya, U. A. Spectrochim. Acta. 1993, 49A, 685-690 DOI: https://doi.org/10.1016/0584-8539(93)80091-N
Pal, S.; Gohdes, J. W.; Wilisch, W. C. A.; Armstrong, W. H. Inorg. Chem. 1992, 31, 713-716 DOI: https://doi.org/10.1021/ic00030a036
Gielen, M. Appl. Organomet. Chem. 2002, 16, 481-494 DOI: https://doi.org/10.1002/aoc.331
Sigel, H. Metal Ions in Biological Systems, Marcel Dekker, 1975
Janiak, J. C. J. Chem. Soc. Dalton Trans. 2000, 2000, 3885-3896 DOI: https://doi.org/10.1039/b003010o
Sánchez-Guadarrama, O.; López-Sandoval, H.; Sánchez-Bartez, F.; Gracia-Mora, I.; Hopfl, H.; Barba-Behrens, N. J. Inorg. Biochem. 2009, 103, 1204-1213 DOI: https://doi.org/10.1016/j.jinorgbio.2009.05.018
López-Sandoval, H.; Londono-Lemos, M. E.; Garza-Velasco, R.; Poblano-Meleléndez, I.; Granada-Macías, P.; Gracia-Mora, I.; Barba-Behrens, N., J. Inorg. Biochem. 2008, 102, 1267-1276 DOI: https://doi.org/10.1016/j.jinorgbio.2008.01.016
Betanzos-Lara, S.; Gracia-Mora, I.; Granada-Macías, P.; Flores-Álamo, M.; Barba- Behrens, N. Inorg. Chim. Acta. 2013, 397, 94-100. DOI: https://doi.org/10.1016/j.ica.2012.11.026
Betanzos-Lara, S.; Chmel, N. P.; Zimmerman, M. T.; Barrón-Sosa, L. R.; Garino, C.; Salassa L.; Rodger, A.; Brumaghim, J. L.; Gracia-Mora, I.; Barba-Behrens, N. Dalton Trans., 2015, 44, 3673-3685. DOI: https://doi.org/10.1039/C4DT02883J

Downloads
Additional Files
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









