Electrocatalytic Production of Hydrogen Gas by a Cobalt Formamidinate Complex
DOI:
https://doi.org/10.29356/jmcs.v63i3.535Keywords:
cobalt, formamidinate, electrocatalytic, hydrogen gasAbstract
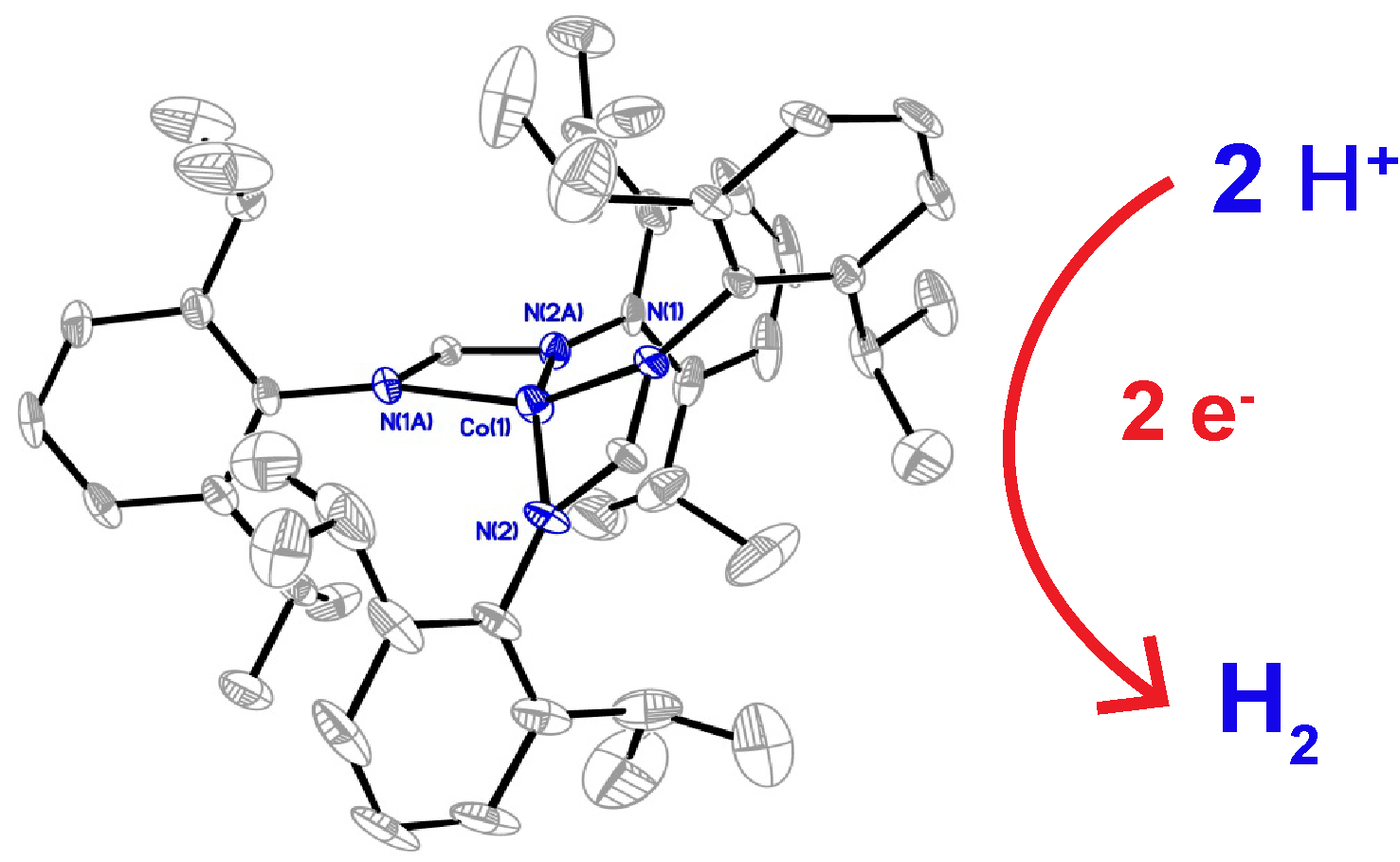
ABSTRACT. A molecular cobalt complex, Co(DippF)2 (where DippF is the anion of N,N’-bis[2,6-diisopropylphenyl]-formamidine), (1),is able to electrochemically produce hydrogen gas from the reduction of organic acids in homogeneous solutions. Compound 1 has a distorted square planar structure as evidenced through X-ray crystallography studies, and an effective magnetic moment of 4.13, obtained by the Evans method, that corresponds to three unpaired electrons. Compound 1 shows an irreversible cathodic peak at –1.59 V vs Fc/Fc+ which is assigned to the reduction of CoII to CoI. In the presence of organic acids the onset of catalytic current is observed at –1.2 V, –1.45 V and –1.89 V vs. Fc/Fc+ with p-toluenesulfonic acid, benzoic acid and phenol as the proton source, respectively, in MeCN as the solvent. Detection of hydrogen gas was obtained by GC-MS with Faradaic efficiencies ranging from 85% to 100%. Kinetic studies using foot-of-the-wave analysis (FOWA) reveal a linear dependence of the observed rate constant, kobs, against acid concentration in the range of 0.065 to 10.02 s-1.
Downloads
References
(1) Gray, H. B. Nat. Chem. 2009, 1 (1), 7–7. DOI: https://doi.org/10.1038/nchem.141
(2) Cook, T. R.; Dogutan, D. K.; Reece, S. Y.; Surendranath, Y.; Teets, T. S.; Nocera, D. G. Chem. Rev. 2010, 110 (11), 6474–6502. DOI: https://doi.org/10.1021/cr100246c
(3) Lewis, N. S.; Nocera, D. G. Proc. Natl. Acad. Sci. 2006, 103 (43), 15729–15735. DOI: https://doi.org/10.1073/pnas.0603395103
(4) Sanchez, J.; Ramos-Garcés, M. V.; Narkeviciute, I.; Colón, J. L.; Jaramillo, T. F. Catalysts 2017, 7 (5), 132. DOI: https://doi.org/10.3390/catal7050132
(5) Wang Lihuan; Tranca Diana C.; Zhang Jian; Qi Yanpeng; Sfaelou Stavroula; Zhang Tao; Dong Renhao; Zhuang Xiaodong; Zheng Zhikun; Seifert Gotthard. Small 2017, 13 (37), 1700783. DOI: https://doi.org/10.1002/smll.201700783
(6) Turner, J. A. Science 2004, 305 (5686), 972–974. DOI: https://doi.org/10.1126/science.1103197
(7) Thoi, V. S.; Sun, Y.; Long, J. R.; Chang, C. J. Chem. Soc. Rev. 2013, 42 (6), 2388–2400. DOI: https://doi.org/10.1039/C2CS35272A
(8) Popczun, E. J.; Read, C. G.; Roske, C. W.; Lewis, N. S.; Schaak, R. E. Angew. Chem. Int. Ed Engl. 2014, 53 (21), 5427–5430. DOI: https://doi.org/10.1002/anie.201402646
(9) Wang, M.; Chen, L.; Sun, L. Energy Environ. Sci. 2012, 5 (5), 6763–6778. DOI: https://doi.org/10.1039/c2ee03309g
(10) Faber, M. S.; Lukowski, M. A.; Ding, Q.; Kaiser, N. S.; Jin, S. J. Phys. Chem. C 2014, 118 (37), 21347–21356. DOI: https://doi.org/10.1021/jp506288w
(11) Chen, X.; Wang, D.; Wang, Z.; Zhou, P.; Wu, Z.; Jiang, F. Chem. Commun. 2014, 50 (79), 11683–11685. DOI: https://doi.org/10.1039/C4CC05936K
(12) Bediako, D. K.; Solis, B. H.; Dogutan, D. K.; Roubelakis, M. M.; Maher, A. G.; Lee, C. H.; Chambers, M. B.; Hammes-Schiffer, S.; Nocera, D. G. Proc. Natl. Acad. Sci. U. S. A. 2014, 111 (42), 15001–15006. DOI: https://doi.org/10.1073/pnas.1414908111
(13) Bayati, M. Chemcatchem 2017, 9. DOI: https://doi.org/10.1002/cctc.201700744
(14) Patra, B. C.; Khilari, S.; Manna, R. N.; Mondal, S.; Pradhan, D.; Pradhan, A.; Bhaumik, A. ACS Catal. 2017, 7 (9), 6120–6127. DOI: https://doi.org/10.1021/acscatal.7b01067
(15) Yap, C. P.; Poh, H. T.; Fan, W. Y. RSC Adv. 2016, 6 (7), 5903–5906. DOI: https://doi.org/10.1039/C5RA23887K
(16) Zheng, Y.; Jiao, Y.; Zhu, Y.; Li, L. H.; Han, Y.; Chen, Y.; Du, A.; Jaroniec, M.; Qiao, S. Z. Nat. Commun. 2014, 5, ncomms4783. DOI: https://doi.org/10.1038/ncomms4783
(17) Wu, Y.; Zarei-Chaleshtori, M.; Torres, B.; Akter, T.; Diaz-Moreno, C.; Saupe, G. B.; Lopez, J. A.; Chianelli, R. R.; Villagrán, D. Int. J. Hydrog. Energy 2017, 42 (32), 20669–20676. DOI: https://doi.org/10.1016/j.ijhydene.2017.07.028
(18) Solis, B. H.; Hammes-Schiffer, S. J. Am. Chem. Soc. 2011, 133 (47), 19036–19039. DOI: https://doi.org/10.1021/ja208091e
(19) Matson, B. D.; Peters, J. C. ACS Catal. 2018, 8 (2), 1448–1455. DOI: https://doi.org/10.1021/acscatal.7b03068
(20) McCrory, C. C. L.; Uyeda, C.; Peters, J. C. J. Am. Chem. Soc. 2012, 134 (6), 3164–3170. DOI: https://doi.org/10.1021/ja210661k
(21) Marinescu, S. C.; Winkler, J. R.; Gray, H. B.Proc. Natl. Acad. Sci. 2012, 109 (38), 15127–15131. DOI: https://doi.org/10.1073/pnas.1213442109
(22) Solis, B. H.; Maher, A. G.; Dogutan, D. K.; Nocera, D. G.; Hammes-Schiffer, S. Proc. Natl. Acad. Sci. 2016, 113 (3), 485–492. DOI: https://doi.org/10.1073/pnas.1521834112
(23) Hu, X.; Brunschwig, B. S.; Peters, J. C. J. Am. Chem. Soc. 2007, 129 (29), 8988–8998. DOI: https://doi.org/10.1021/ja067876b
(24) Bhugun, I.; Lexa, D.; Savéant, J.-M.J. Am. Chem. Soc. 1996, 118 (16), 3982–3983. DOI: https://doi.org/10.1021/ja954326x
(25) Lee, C. H.; Dogutan, D. K.; Nocera, D. G. J. Am. Chem. Soc. 2011, 133 (23), 8775–8777. DOI: https://doi.org/10.1021/ja202136y
(26) Kaeffer, N.; Chavarot-Kerlidou, M.; Artero, V. Acc. Chem. Res. 2015, 48 (5), 1286–1295. DOI: https://doi.org/10.1021/acs.accounts.5b00058
(27) Mondal, B.; Sengupta, K.; Rana, A.; Mahammed, A.; Botoshansky, M.; Dey, S. G.; Gross, Z.; Dey, Inorg. Chem. 2013, 52 (6), 3381–3387. DOI: https://doi.org/10.1021/ic4000473
(28) Beyene, B. B.; Mane, S. B.; Hung, C.-H. Chem. Commun. 2015, 51 (81), 15067–15070. DOI: https://doi.org/10.1039/C5CC05582B
(29) Fihri, A.; Artero, V.; Razavet, M.; Baffert, C.; Leibl, W.; Fontecave, M. Angew. Chem. Int. Ed Engl. 2008, 47 (3), 564–567. DOI: https://doi.org/10.1002/anie.200702953
(30) Jurss, J. W.; Khnayzer, R. S.; Panetier, J. A.; Roz, K. A. E.; Nichols, E. M.; Head-Gordon, M.; Long, J. R.; Castellano, F. N.; Chang, C. J. Chem. Sci. 2015, 6 (8), 4954–4972. DOI: https://doi.org/10.1039/C5SC01414J
(31) Chen, L.; Khadivi, A.; Singh, M.; Jurss, J. W. Inorg. Chem. Front. 2017, 4 (10), 1649–1653. DOI: https://doi.org/10.1039/C7QI00362E
(32) Elkin, T.; Kulkarni, N. V.; Tumanskii, B.; Botoshansky, M.; Shimon, L. J. W.; Eisen, M. S. Organometallics 2013, 32 (21), 6337–6352. DOI: https://doi.org/10.1021/om4006998
(33) Evans, D. F. 400.J. Chem. Soc. Resumed 1959, 0 (0), 2003–2005. DOI: https://doi.org/10.1039/jr9590002003
(34) Ostfeld, D.; Cohen, I. A. J. Chem. Educ. 1972, 49 (12), 829. DOI: https://doi.org/10.1021/ed049p829
(35) Costentin, C.; Drouet, S.; Robert, M.; Savéant, J.-M. J. Am. Chem. Soc. 2012, 134 (27), 11235–11242. DOI: https://doi.org/10.1021/ja303560c
(36) Rountree, E. S.; McCarthy, B. D.; Eisenhart, T. T.; Dempsey, J. L. Inorg. Chem. 2014, 53 (19), 9983–10002. DOI: https://doi.org/10.1021/ic500658x
(37) Rountree, E. S.; Martin, D. J.; McCarthy, B. D.; Dempsey, J. L. ACS Catal. 2016, 6 (5), 3326–3335. DOI: https://doi.org/10.1021/acscatal.6b00667
(38) Yang, L.; Powell, D. R.; Houser, R. P. Dalton Trans. Camb. Engl. 2003 2007, No. 9, 955–964. DOI: https://doi.org/10.1039/B617136B
(39) Maher, A. G.; Passard, G.; Dogutan, D. K.; Halbach, R. L.; Anderson, B. L.; Gagliardi, C. J.; Taniguchi, M.; Lindsey, J. S.; Nocera, D. G. ACS Catal. 2017, 7 (5), 3597–3606. DOI: https://doi.org/10.1021/acscatal.7b00969
(40) Fryzuk, M. D.; Leznoff, D. B.; Thompson, R. C.; Rettig, S. J. J. Am. Chem. Soc. 1998, 120 (39), 10126–10135. DOI: https://doi.org/10.1021/ja9721632
(41) Carabineiro, S. A.; Silva, L. C.; Gomes, P. T.; Pereira, L. C. J.; Veiros, L. F.; Pascu, S. I.; Duarte, M. T.; Namorado, S.; Henriques, R. T. Inorg. Chem. 2007, 46 (17), 6880–6890. DOI: https://doi.org/10.1021/ic062125w
(42) Fink, K.; Wang, C.; Staemmler, V. Inorg. Chem. 1999, 38 (17), 3847–3856. DOI: https://doi.org/10.1021/ic990280n
(43) Gerloch, M.; Quested, P. N. J. Chem. Soc. Inorg. Phys. Theor. 1971, 0 (0), 3729–3741. DOI: https://doi.org/10.1039/j19710003729

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









