Evidence of Radical Intermediate Generated in the Electrochemical Oxidation of Iodide
DOI:
https://doi.org/10.29356/jmcs.v63i3.529Keywords:
Inner sphere, iodide oxidation, dye-sensitized solar cell, two electron oxidationAbstract
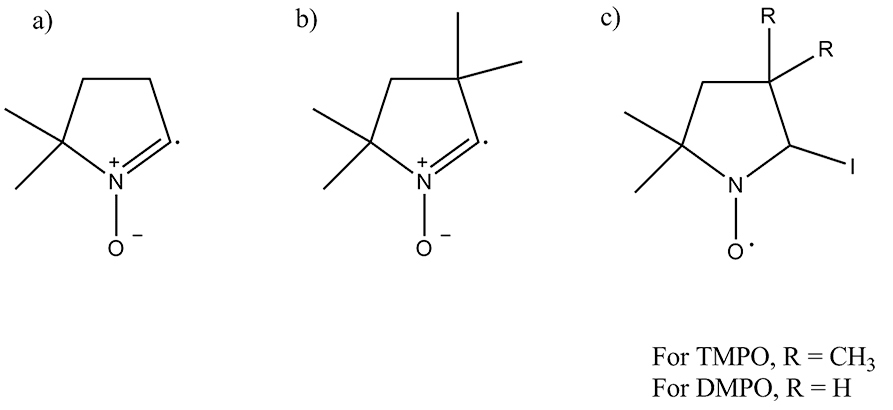
We present evidence of the generation of radical ion formation during the oxidation of iodide on a fluorine doped tin oxide (FTO) electrode in acetonitrile. The cyclic voltammograms for the oxidation of iodide and triiodide on FTO are significantly different as in the case of the oxidation of Pt electrode. These differences are assigned to kinetic differences on the FTO surface that require significant over potentials to drive the oxidation of iodide and triiodide. We propose that at the highly positive potentials the iodine radical intermediate, I·, becomes thermodynamically stable at FTO. The radical nature of the intermediate was verified by the formation of radicals of the usual traps of 5,5-dimethyl-1-pyrroline N-oxide (DMPO) and 2,2,5,5 tetramethyl-1-pyrroline N-oxide (TMPO) when these were added to an electrolyzed solution. Irradiation of an iodine solution causes the homolytic cleavage of I2 and yields the same radical intermediate with TMPO as in the electrolysis experiment. Similar results were obtained from the electrolysis of bromide solutions upon addition of TMPO. Short term electrolysis (< 1 h) gives triiodide as a final product while long-term electrolysis (> 17 h) yields additional byproducts. Byproducts were determined to be organoiodines by gas chromatography-mass spectrometry (GC-MS). Overall, our results are consistent with iodine atoms reacting with the electrolyte during electrolysis at the FTO electrode and with a sequential two-electron transfer process.
Downloads
References
Evans, D. H. Chem. Rev. 2008, 108, 2113-2144 DOI: 10.1021/cr068066l. DOI: https://doi.org/10.1021/cr068066l
Chang, J.; Bard, A. J. J. Am. Chem. Soc. 2013, 136, 311-320 DOI: 10.1021/ja409958a. DOI: https://doi.org/10.1021/ja409958a
Bard, A. J.; Faulkner, L. R. Electrochemical Methods, Fundamentals and Applications; John Wiley and Sons, 2001, p 670.
Gileadi, E. J. Electroanal. Chem. 2002, 532, 181. DOI: https://doi.org/10.1016/S0022-0728(02)00766-0
Khoshtariya, D. E.; Dolidze, T. D.; Zusman, L. D.; Lindbergh, G.; Glaser, J. Inorg. Chem. 2002, 41, 1728-1738 DOI: 10.1021/ic0100525. DOI: https://doi.org/10.1021/ic0100525
Downard, A. J.; Bond, A. M.; Clayton, A. J.; Hanton, L. R.; McMorran, D. A. Inorg. Chem. 1996, 35, 7684-7690 DOI: 10.1021/ic960642g. DOI: https://doi.org/10.1021/ic960642g
Liu, H.; Kuznetsov, A. M.; Masliy, A. N.; Ferguson, J. F.; Korshin, G. V. Environ. Sci. Technol. 2011, 46, 1430-1438 DOI: 10.1021/es203084n. DOI: https://doi.org/10.1021/es203084n
Savéant, J. M. Elements of Molecular and Biomolecular Electrochemistry; Wiley-Interscience: Hoboken, New Jersey, 2006, p 203. DOI: https://doi.org/10.1002/0471758078
Evans, D. H. Chem. Rev. 1998, 52, 194-197 DOI: 10.1021/cr068066l. DOI: https://doi.org/10.3891/acta.chem.scand.52-0194
Gregg, B. A.; Pichot, F.; Ferrere, S.; Fields, C. L. J. Phys. Chem. B 2001, 105, 1422-1429 DOI: 10.1021/jp003000u. DOI: https://doi.org/10.1021/jp003000u
Liu, Y.; Jennings, J. R.; Huang, Y.; Wang, Q.; Zakeeruddin, S. M.; Gra?tzel, M. J. Phys. Chem. C 2011, 115, 18847-18855 DOI: 10.1021/jp204519s. DOI: https://doi.org/10.1021/jp204519s
Li, D.; Li, H.; Luo, Y.; Li, K.; Meng, Q.; Armand, M.; Chen, L. Adv. Funct. Mater. 2010, 20, 3358-3365 DOI: 10.1002/adfm.201000150. DOI: https://doi.org/10.1002/adfm.201000150
Lee, J.; Lee, C.; Lee, Y.; Cho, K.; Choi, J.; Park, J.-K. J. Solid State Electrochem. 2012, 16, 657-663 DOI: 10.1007/s10008-011-1405-9. DOI: https://doi.org/10.1007/s10008-011-1405-9
Tian, H.; Sun, L. J. Mater. Chem. 2011, 21, 10592-10601 DOI: 10.1039/c1jm10598a. DOI: https://doi.org/10.1039/c1jm10598a
Hattori, S.; Wada, Y.; Yanagida, S.; Fukuzumi, S. J. Am. Chem. Soc. 2005, 127, 9648-9654 DOI: 10.1021/ja0506814. DOI: https://doi.org/10.1021/ja0506814
Hagfeldt, A.; Grätzel, M. Acc. Chem. Res. 2000, 33, 269-277 DOI: 10.1021/ar980112j. DOI: https://doi.org/10.1021/ar980112j
Ardo, S.; Meyer, G. J. Chem. Soc. Rev. 2009, 38, 115-164 DOI: 10.1039/b804321n. DOI: https://doi.org/10.1039/B804321N
Gardner, J. M.; Abrahamsson, M.; Farnum, B. H.; Meyer, G. J. J. Am. Chem. Soc. 2009, 131, 16206-16214 DOI: 10.1021/ja905021c. DOI: https://doi.org/10.1021/ja905021c
Gardner, J. M.; Giaimuccio, J. M.; Meyer, G. J. J. Am. Chem. Soc. 2008, 130, 17252-17253 DOI: 10.1021/ja807703m. DOI: https://doi.org/10.1021/ja807703m
Rowley, J.; Meyer, G. J. J. Phys. Chem. C 2009, 113, 18444-18447 DOI: 10.1021/jp907265x. DOI: https://doi.org/10.1021/jp907265x
Rowley, J. G.; Farnum, B. H.; Ardo, S.; Meyer, G. J. J. Phys. Chem. Lett. 2010, 1, 3132-3140 DOI: 10.1021/jz101311d. DOI: https://doi.org/10.1021/jz101311d
Popov, A. I.; Geske, D. H. J. Am. Chem. Soc. 1958, 80, 1340-1352 DOI: 10.1021/ja01539a018. DOI: https://doi.org/10.1021/ja01539a018
Macagno, V. A.; Giordano, M. C.; Arvía, A. J. Electrochim. Acta 1969, 14, 335-357 DOI: http://dx.doi.org/10.1016/0013-4686(69)85005-X. DOI: https://doi.org/10.1016/0013-4686(69)85005-X
Nakata, R.; Okazaki, S.; Fujinaga, T. J. Electroanal. Chem. 1981, 125, 413-420 DOI: http://dx.doi.org/10.1016/S0022-0728(81)80358-0. DOI: https://doi.org/10.1016/S0022-0728(81)80358-0
Rogers, E. I.; Streeter, I.; Aldous, L.; Hardacre, C.; Compton, R. G. J. Phys. Chem. C 2008, 112, 10976-10981 DOI: 10.1021/jp802934y. DOI: https://doi.org/10.1021/jp802934y
Nelson, I. V.; Iwamoto, R. T. J. Electroanal. Chem. 1964, 7, 218-221 DOI: http://dx.doi.org/10.1016/0022-0728(64)80015-2. DOI: https://doi.org/10.1016/0022-0728(64)80015-2
Bourdillon, C.; Demaille, C.; Moiroux, J.; Saveant, J.-M. J. Am. Chem. Soc. 1995, 117, 11499-11506 DOI: 10.1021/ja00151a013. DOI: https://doi.org/10.1021/ja00151a013
Eberson, L. J. Chem. Soc., Perkin Trans. 2 1994, 171-176 DOI: 10.1039/p29940000171. DOI: https://doi.org/10.1039/p29940000171
Electron paramagnetic resonance: A practicioner's toolkit; John Wiley and Sons, Inc.: Hoboken, New Jersey, 2009.
Duling, D. R. Journal of Magnetic Resonance, Series B 1994, 104, 105-110 DOI: https://doi.org/10.1006/jmrb.1994.1062.
Wang, Q.; Rodríguez-López, J.; Bard, A. J. J. Am. Chem. Soc. 2009, 131, 17046-17047 DOI: 10.1021/ja907626t. DOI: https://doi.org/10.1021/ja907626t
Frontana-Uribe, B. A.; Little, R. D.; Ibanez, J. G.; Palma, A.; Vasquez-Medrano, R. Green Chem. 2010, 12, 2099-2119 DOI: 10.1039/C0GC00382D. DOI: https://doi.org/10.1039/c0gc00382d
Yan, M.; Kawamata, Y.; Baran, P. S. Chem. Rev. 2017, 117, 13230-13319 DOI: 10.1021/acs.chemrev.7b00397. DOI: https://doi.org/10.1021/acs.chemrev.7b00397
Ibanez, J. G.; Frontana-Uribe, B. A.; Vasquez-Medrano, R. J. Mex. Chem. Soc. 2016, 60, 247-260.

Downloads
Additional Files
Published
Issue
Section
License
Copyright (c) 2019 Mario A Alpuche Aviles

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









