Microwave Assisted One Pot Synthesis of Tetrazole Based 3-hydroxy-4H-chromen-4-ones by modified Algar-Flynn-Oyamada reaction and their Antimicrobial activity
DOI:
https://doi.org/10.29356/jmcs.v63i4.448Keywords:
2-hydroxy acetophenone, 4-(1H-tetrazol-5-yl) benzaldehyde, microwave irradiation, antimicrobial activity.Abstract
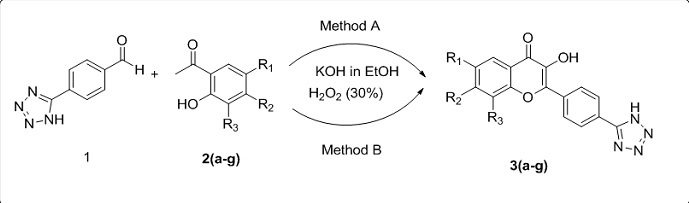
In the present work, we report the one pot synthesis of tetrazole based 3-hydroxy-4H-chromen-4-ones 3(a-g) from 4-(1H-tetrazol-5-yl)benzaldehyde and 2-hydroxy acetophenone using KOH and H2O2 by modified Algar-Flynn-Oyamada reaction under conventional and microwave irradiation conditions. In this technique, flavonols are synthesized without isolating chalcones, in good yields. All the synthesized compounds were characterized by IR, NMR, MS and elemental. All newly synthesized compounds were screened for their in-vitro antimicrobial activity against strains such as Staphylococcus aurous, Bacillus subtilis, Klebsiella pneumonia, Escherichia coli, Aspergillus Niger, Aspergillus flavus, and Fusarium oxysporum. The results of antimicrobial studies revealed that most of the compounds exhibit good activity.
Downloads
References
1. Genoux, E.; Nicolle, E.; Boumendjel, A. Curr. Org. Chem. 2011, 15, 2608-2615 DOI: https://doi.org/10.2174/138527211796367363
Xiao, Z. P.; Peng, Z. Y.; Peng, M. J.; Yan, W. B.; Ouyang, Y. Z.; Zhu, H. L. Mini-Rev. Med. Chem. 2011, 11, 169-177 DOI: 10.2174/138955711794519546 DOI: https://doi.org/10.2174/138955711794519546
Franke, A. A.; Cooney, R. V.; Custer, L. J.; Mordan, L. J.; Tanaka, Y. Adv. Exp. Med. Biol. 1998, 439, 237-248 DOI: https://doi.org/10.1007/978-1-4615-5335-9_17
Ashok, D.; Kifah, M. A.; Lakshmi, B. V.; Sarasija, M.; Adam, S. Chem. Heterocycl. Compd. 2016, 52, 172-176 DOI: 10.1007/s10593-016-1852-4 DOI: https://doi.org/10.1007/s10593-016-1852-4
Chohan, Z. H.; Rauf, A.; Naseer, M. M.; Somra, M. A.; Supuran, C. T. J. Enzyme Inhib. Med. Chem. 2006, 21, 173-177 DOI: https://doi.org/10.1080/14756360500533059
Kim, H. P.; Son, K. H.; Chang, H. W.; Kang, S. S., Nat. Prod. Sci., 1996, 2, 1-8
Ercelen, S.; Klymchenko, A. S.; Demchenko, A. P. Anal. Chim. Acta., 2002, 464 (2), 273-287 DOI: 10.1016/S0003-2670(02)00493-2 DOI: https://doi.org/10.1016/S0003-2670(02)00493-2
Algar, J. Flynn, J. P. Proc. Roy. Irish Acad., 1934, 42B, 1-18
Oyamada, T. Bull. Chem. Soc. Jopan, 1935, 10, 182-186 DOI: https://doi.org/10.1246/bcsj.10.182
Murakami, M.; Irie, T. Proc. Imp. Acad. Tokyo 1935, 11, 229 DOI: https://doi.org/10.2183/pjab1912.11.229
Reichel, L.; Steudel, J. Liebigs Ann. 1942, 553, 83-97 DOI: https://doi.org/10.1002/jlac.19425530105
Ostrovskii, V. A.; Koldobskii, G. I.; Trifonov, R. E. Comp. Heterocycl. Chem. III., 2008, 6, 257-423 DOI: https://doi.org/10.1016/B978-008044992-0.00517-4
Srinivas, B.; Kumar, P. V.; Reddy, P. N.; Venu, S.; Shyam, P., Krupadanam, G. D. Russ J Bioorg Chem., 2018, 44, 244 DOI: https://doi.org/10.1134/S1068162018020097
Mulwad, V. V.; Pawar, R. B.; Chaskar, A. C. J. Korean Chem. Soc., 2008, 52, 249-256 DOI: 10.5012/jkcs.2008.52.3.249 DOI: https://doi.org/10.5012/jkcs.2008.52.3.249
Kumar, S. M.; Manjunath, B. C.; Lingaraju, G. S.; Abdoh, M. M. M.; Sadashiva, M. P.; Lokanath, N. K., The FASEB Journal., 2007, 21, 790 DOI:10.4236/csta.2013.23017 DOI: https://doi.org/10.4236/csta.2013.23017
Bhaskar, V. H.; Mohite, P. B., J. Optoelectron. Biomed. Mater., 2010, 2, 249-259 DOI: https://doi.org/10.31826/9781463228903-017
Le Bourdonnec, B.; Meulon, E.; Yous, S.; Goossens, J. F.; Houssin, R.; Hénichart, J. P. J. Med. Chem., 2000, 43, 2685 DOI: 10.1021/jm9904147 DOI: https://doi.org/10.1021/jm9904147
Adamec, J.; Waisser, K.; Kuneš, J.; Kaustová. J. Archiv der Pharmazie, 2005, 338(8), 385-389 DOI: https://doi.org/10.1002/ardp.200400967
Upadhayaya, R. S.; Jain, S.; Sinha, N.; Kishore, N.; Chandra, R.; Arora, S. K. Eur. J. Med. Chem., 2004, 39, 579 DOI: 10.1016/j.ejmech.2004.03.004 DOI: https://doi.org/10.1016/j.ejmech.2004.03.004
Rajasekaran, A.; Murugesan, S.; Ananda Rajagopal, K. Arch of Pharmacal Res., 2006, 29, 535-540 DOI: https://doi.org/10.1007/BF02969261
Ashok, D.; Rangu, K.; Gundu, S.; Lakkadi, A.; Tigulla, P. Med. Chem. Res., 2017, 26, 1735 DOI: https://doi.org/10.1007/s00044-017-1834-9
Ashok, D.; Vijaya Lakshmi, B.; Ravi, S.; Ganesh, A. Med. Chem. Res., 2015, 24, 1487 DOI: 10.1007/s00044-014-1204-9 DOI: https://doi.org/10.1007/s00044-014-1204-9
Akhlaghinia, B.; Soodabeh Rezazadeh, J. Braz. Chem. Soc., 2012, 23, 2197 DOI: 10.1590/S0103 DOI: https://doi.org/10.1590/S0103-50532013005000005
Ashok, D.; Ravi, S.; Vijaya Lakshmi, B.; Ganesh, A., J. Serb. Chem. Soc., 2015, 80, 1 DOI: 10.2298/JSC141203051A DOI: https://doi.org/10.2298/JSC140021101A
Serdiuk, I. E.; Roshal, A. D.; B?a?ejowski, J. Chem. Heterocycl. Compd., 2014, 50, 396 DOI: https://doi.org/10.1007/s10593-014-1487-2

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









