Effect of the thermal annealing on the phase transitions of biogenic CaCO3 nanostructures
DOI:
https://doi.org/10.29356/jmcs.v63i1.422Keywords:
Keywords, Calcite, aragonite and portlandite powders, CaCO3 rhombohedral nanoparticle, (Ca(OH)2) hexagonal phase, CaCO3 biogenic compoundAbstract
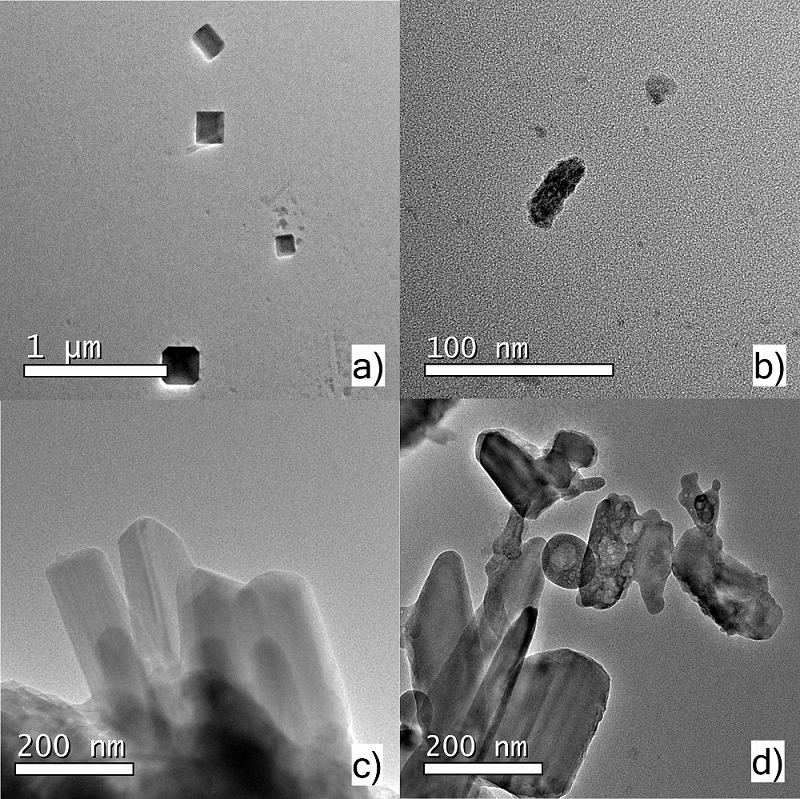
The issue of the present research lays its foundation on the proposal of the Crassostrea virginica waste oyster shells (WOS) reuse to obtain calcium carbonate powder (CaCO3) and calcium hydroxide (Ca(OH)2) nanostructured, using thermal annealing treatments. The oysters shells were subjected to a previous physical grinding process to decrease their size (smaller sizes 0.074 mm). The parameter studied was the effect of annealing temperature (500, 700 and 900 °C in air atmosphere) on the structural properties and morphology of the powders by FTIR, XRD, SEM and HRTEM. The X-ray diffraction results indicate that the WOS in their natural state and thermally annealed at 500 °C have two phases of CaCO3 the rhombohedral form for calcite with crystallite size around 24 nm and aragonite traces in orthorhombic phase. At 700 °C, the WOS powder is transformed into calcium hydroxide, also known as portlandite (Ca(OH)2), attributed to the absorption of water released during the thermal decomposition of CaCO3. This crystalline phase does not change when the temperature increases to 900 °C. The SEM and HRTEM analysis of WOS powders reveals that with a thermal annealing treatment it is possible to obtain nanostructured CaCO3. FTIR analysis demonstrates the biogenic origin of CaCO3, due to amide groups. The nanostructured CaCO3 obtained by grinding and thermal annealing of WOS, can be used as drying agent, or as additive in ceramic and glass.
The issue of the present research lays its foundation on the proposal of the Crassostrea virginica waste oyster shells (WOS) reuse to obtain calcium carbonate powder (CaCO3) and calcium hydroxide (Ca(OH)2) nanostructured, using thermal annealing treatments. The oysters shells were subjected to a previous physical grinding process to decrease their size (smaller sizes 0.074 mm). The parameter studied was the effect of annealing temperature (500, 700 and 900 °C in air atmosphere) on the structural properties and morphology of the powders by FTIR, XRD, SEM and HRTEM. The X-ray diffraction results indicate that the WOS in their natural state and thermally annealed at 500 °C have two phases of CaCO3 the rhombohedral form for calcite with crystallite size around 24 nm and aragonite traces in orthorhombic phase. At 700 °C, the WOS powder is transformed into calcium hydroxide, also known as portlandite (Ca(OH)2), attributed to the absorption of water released during the thermal decomposition of CaCO3. This crystalline phase does not change when the temperature increases to 900 °C. The SEM and HRTEM analysis of WOS powders reveals that with a thermal annealing treatment it is possible to obtain nanostructured CaCO3. FTIR analysis demonstrates the biogenic origin of CaCO3, due to amide groups. The nanostructured CaCO3 obtained by grinding and thermal annealing of WOS, can be used as drying agent, or as additive in ceramic and glass.
Downloads
References
Yu, Y.; Smyth J. R.; Bon, P. American Mineralogist. 2012, 97, 707–712. DOI: http://dx.doi.org/10.2138/am.2012.3923 DOI: https://doi.org/10.2138/am.2012.3923
Yoshioka S.; Kitano Y. Geochemical Journal. 1985, 19, 245 to 249. DOI: https://doi.org/10.2343/geochemj.19.245
Manjusha, H.; Neethumol, V.; Benny, C. A.; Sreenivasan P.V.; Jenish, P.; Asmy, A.K.A, International Journal of Scientific and Research Publications. 2014, 4, 10. ISSN 2250-3153
Checa, A.G.; Esteban-Delgado, F.J.; Rodr?íguez-Navarro, A.B. Journal of Sctructural Biology. 2007, 157, 393–402. DOI:10.1016/j.jsb.2006.09.005 DOI: https://doi.org/10.1016/j.jsb.2006.09.005
Hamester, M.R.R.; Balzer, P.S.; Becker, D. Materials Research. 2012, 15, 204-208. DOI: http://dx.doi.org/10.1590/S1516-14392012005000014 DOI: https://doi.org/10.1590/S1516-14392012005000014
Chen Y.; Lian X.; Li Z.; Zheng S., Wang Z., Advanced Powder Technology, 2015, 26, 505-510. DOI: http://dx.doi.org/10.1016/j.apt.2014.12.007 DOI: https://doi.org/10.1016/j.apt.2014.12.007
Bastida J., Bolós C., Pardo P., Serrano F. J., Bol. Soc. Esp. Ceram. V. 2004, 43, 80-83. DOI: 10.3989/cyv.2004.v43.i1.621 DOI: https://doi.org/10.3989/cyv.2004.v43.i1.621
Yoon, G.L.; Kim, B.T.; Kim, B.O.;Han, S.H. Waste Managment, 2003, 23, 825-34. DOI: 10.1016/S0956-053X(02)00159-9 DOI: https://doi.org/10.1016/S0956-053X(02)00159-9
Yong, S. O.; Sang Eun, O.; Mahtab, A.; Hyun, S.; Kwon Rae, K.; Deok Hyun, M.; Sang Soo, L.; Kyoung Jae, L.; Weon Tai, J.; Jae E, Y. Environ Earth Science. 2010, 61,1301-1308. DOI 10.1007/s12665-010-0674-4
Lee, C.W.; Kwon, H.B.; Jeon, H.P.; Koopman, B. Journal Clean Production. 2009, 17,683–687. DOI : 10.1016/j.jclepro.2008.11.019 DOI: https://doi.org/10.1016/j.jclepro.2008.11.019
Jae-Hoon Huh, Young-Hoon Choi*, Chilakala Ramakrishna, Sun Hee Cheong** and Ji Whan Ahn Jae-Hoon, H., Young-Hoon, C., Chilakala, R., Sun Hee, C., Ji Whan, A. Journal of the Korean Ceramic Society. 2016, 53, 429-434. DOI: http://dx.doi.org/10.4191/kcers.2016.53.4.429. DOI: https://doi.org/10.4191/kcers.2016.53.4.429
Khan, MD., Ahn, JW., Nam, G. Journal of Environmental Management. 2018, 223, 947-951. DOI: 10.1016/j.jenvman.2018.07.011 DOI: https://doi.org/10.1016/j.jenvman.2018.07.011
Weiner, S., Mahamid, J., Politi, Y., Ma, Y., Addadi, L. Frontiers of Materials Science in China. 2009, 3, 104. DOI: 10.1007/s11706-009-0036-x. DOI: https://doi.org/10.1007/s11706-009-0036-x
Cartwright, J.H. E., Checa, A.G., Gale, J.D., Gebauer, D., Sainz?Díaz, C. I. Angewandte Chemie. 2012, 51, 11960-11970. DOI: https://doi.org/10.1002/anie.201203125.
Kladi A., Klepetsanis P.G., Østvold T., Kontoyiannis C.G., Koutsoukos P.G. Advances in Crystal Growth Inhibition Technologies. 2002, Springer, Boston, MA. DOI: 10.1.1.846.4070&rep=rep1&type=pdf
Chiou I.J., Chen C.H., Li Y.H. Construction and Building Materials. 2014, 64, 480-487. DOI: http://dx.doi.org/10.1016/j.conbuildmat.2014.04.101 DOI: https://doi.org/10.1016/j.conbuildmat.2014.04.101
Ramon de los Santos, C., Barajas Fernandez, J., Perez Hernandez, G., Hernandez Rivera, M.A., Diaz Flores, L.L. Bol. Soc. Esp. Cerám. Vidr. 2018, https://doi.org/10.1016/j.bsecv.2018.05.003
Yong, S. O., Jung, E. L., Deok, H. M. Environ Geochem Health. 2011, 33,83-91. DOI 10.1007/s10653-010-9329-3 DOI: https://doi.org/10.1007/s10653-010-9329-3
Islam, K.N.; Abu Bakar, Z.B.; Ali, E.; Bin Hussein, M.Z.; Noordin, M.M.; Loqman, M.Y.; Miah, G.; Wahid, H.; Hashim, U. Powder Technology. 2013,235, 70-75. DOI: https://doi.org/10.1016/j.powtec.2012.09.041
Yash, B.; Vishnu, K. P.; Jian, L.Journal of Materials Chemistry. 2016, 2,14270-14288. DOI:10.1039/C4TA02070G DOI: https://doi.org/10.1039/C4TA02070G
Shan-Yang, L.; Mei-Jane, L.;Wen-Ting, C. Spectroscopy. 2007, 21, 1-30. DOI: http://dx.doi.org/10.1155/2007/278765 DOI: https://doi.org/10.1155/2007/278765
Yousefpour, M.; Askari, N.; Abdollah-Pour, H. Biotechnology and Biomaterials. 2011, 1,1-4. DOI:10.4172/2155-952X.1000105 DOI: https://doi.org/10.4172/2155-952X.1000105
Vagenas, N.V.; Gatsouli, A.;Kontoyannis, C.G. Talanta. 2003, 10, 831-836. DOI: 10.1016/S0039-9140(02)00638-0. DOI: https://doi.org/10.1016/S0039-9140(02)00638-0
Rodríguez-Navarro, C.; Elert, K.; Sevcik, R. Cryst. Eng. Comm. 2016, 18, 6594-6607. DOI:10.1039/C6CE01202G DOI: https://doi.org/10.1039/C6CE01202G

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









