Air-Stable Triazole-Based Ru(II) Complexes Catalyzed Transfer Hydrogenation of Ketones and Aldehydes Using Ethanol as a Solvent and a Hydrogen Donor
DOI:
https://doi.org/10.29356/jmcs.v68i4.2308Keywords:
catalysis, transfer hydrogenation, ruthenium, triazole, ethanolAbstract
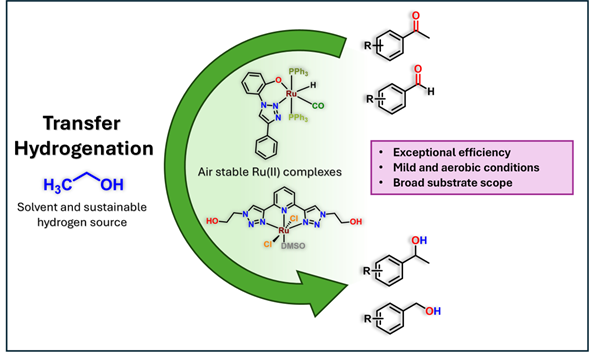
The synthesis and characterization of two air-stable ruthenium (II) complexes from readily available triazole-based ligands are described. Both ruthenium complexes, one bearing a bidentate ligand (C-1) and the other a tridentate ligand (C-2), were tested as catalysts in the transfer hydrogenation of ketones and aldehydes using ethanol as a sustainable hydrogen source under aerobic conditions. Notably, the C-2 complex displayed exceptional efficiency under relatively mild conditions, demonstrating a wide substrate tolerance encompassing both alkyl and aryl ketones, as well as aryl aldehydes. Furthermore, our findings highlight the potential of Ru(II) complexes as effective catalysts for the hydrogenation of carbonyl bonds using ethanol, representing a green and sustainable approach without the necessity for an inert gas.
Resumen. En este trabajo se describe la síntesis y caracterización de dos complejos de rutenio(II) estables al aire con ligantes basados en triazoles. En general, los triazoles pueden obtenerse fácilmente a través de reacciones simples utilizando reactivos comercialmente disponibles. Ambos complejos de rutenio, uno con un ligante bidentado (C-1) y el otro con un ligando tridentado (C-2), se probaron como catalizadores en reacciones de hidrogenación por transferencia de cetonas y aldehídos, utilizando etanol como fuente sostenible de hidrógeno en condiciones aeróbicas. En particular, el complejo C-2 mostró una eficiencia excepcional en condiciones relativamente suaves, demostrando una amplia tolerancia tanto con cetonas alquílicas como aromáticas, además de hidrogenar eficientemente aldehídos aromáticos. Estos resultados ponen de manifiesto el potencial de los complejos de Ru(II) como catalizadores eficaces para la hidrogenación de enlaces carbonilo utilizando etanol, lo que representa un enfoque ecológico y sostenible sin necesidad de un gas inerte.
Downloads
References
Wang, D.; Astruc, D. Chem. Rev. 2015, 115, 6621–6686. DOI: https://doi.org/10.1021/acs.chemrev.5b00203.
Taleb, B.; Jahjah, R.; Cornu, D.; Bechelany, M.; Al Ajami, M.; Kataya, G.; Hijazi, A.; El-Dakdouki, M. H. Molecules. 2023. DOI: https://doi.org/10.3390/molecules28227541.
Romero, A. H. ChemistrySelect. 2020, 5, 13054–13075. DOI: https://doi.org/https://doi.org/10.1002/slct.202002838.
Robertson, A.; Matsumoto, T.; Ogo, S. Dalton Trans. 2011, 40, 10304–10310. DOI: https://doi.org/10.1039/C1DT10544B.
Ghosh, R.; Jana, N. Ch.; Panda, S.; Bagh, B. ACS Sustain. Chem. Eng. 2021, 9, 4903–4914. DOI: https://doi.org/10.1021/acssuschemeng.1c00633.
Garg, N.; Sarkar, A.; Sundararaju, B. Coord. Chem. Rev. 2021, 433, 213728. DOI: https://doi.org/https://doi.org/10.1016/j.ccr.2020.213728.
Hafeez, J.; Bilal, M.; Rasool, N.; Hafeez, U.; Adnan Ali Shah, S.; Imran, S.; Amiruddin Zakaria, Z. Arabian J. Chem. 2022, 15, 104165. DOI: https://doi.org/https://doi.org/10.1016/j.arabjc.2022.104165.
Hu, Z.-Q.; Li, X.; Liu, L.-X.; Yu, C.-B.; Zhou, Y.-G. J. Org. Chem. 2021, 86, 17453–17461. DOI: https://doi.org/10.1021/acs.joc.1c02156.
Gobbo, A.; Ma, X.; Ciancaleoni, G.; Zacchini, S.; Biancalana, L.; Guelfi, M.; Pampaloni, G.; Nolan, S. P.; Marchetti, F. Eur. J. Inorg. Chem. 2023, 26, e202300078. DOI: https://doi.org/https://doi.org/10.1002/ejic.202300078.
Negrete-Vergara, C.; Vega, A.; Cantero-López, P.; Yáñez, O.; Moya, S. A.; Valdebenito, G.; Parra-Melipan, S.; Aguirre, P. Inorg. Chim. Acta 2024, 568, 122064. DOI: https://doi.org/https://doi.org/10.1016/j.ica.2024.122064.
Wang, F.; Zheng, L.-S.; Lang, Q.-W.; Yin, C.; Wu, T.; Phansavath, P.; Chen, G.-Q.; Ratovelomanana-Vidal, V.; Zhang, X. Chem. Commun. 2020, 56, 3119–3122. DOI: https://doi.org/10.1039/C9CC09793G.
Lin, X.; Wang, Y.; Hu, Y.; Zhu, W.; Dou, X. European J. Org. Chem. 2020, 2020, 1046–1049. DOI: https://doi.org/https://doi.org/10.1002/ejoc.202000049.
Wang, Y.; Chang, Z.; Hu, Y.; Lin, X.; Dou, X. Org. Lett. 2021, 23, 1910–1914. DOI: https://doi.org/10.1021/acs.orglett.1c00341.
Everaert, J.; Leus, K.; Rijckaert, H.; Debruyne, M.; Van Hecke, K.; Morent, R.; De Geyter, N.; Van Speybroeck, V.; Van Der Voort, P.; Stevens, C. V. A Green Chem. 2023, 25, 3267–3277. DOI: https://doi.org/10.1039/D3GC00167A.
Yang, Z.; Cheng, W.; Li, Z. Catal. Commun. 2018, 117, 38–42. DOI: https://doi.org/https://doi.org/10.1016/j.catcom.2018.08.004.
Jiang, X.; Cui, X.; Chen, J.; Liu, Q.; Chen, Y.; Zhou, H. Tetrahedron Lett. 2022, 90, 153627. DOI: https://doi.org/https://doi.org/10.1016/j.tetlet.2021.153627.
Xu, H.; Yang, P.; Chuanprasit, P.; Hirao, H.; Zhou, J. (Steve). Angew. Chem. Int. Ed. 2015, 54 , 5112–5116. DOI: https://doi.org/https://doi.org/10.1002/anie.201501018.
Ruan, S.-H.; Fan, Z.-W.; Zhang, W.-J.; Xu, H.; An, D.-L.; Wei, Z.-B.; Yuan, R.-M.; Gao, J.-X.; Li, Y.-Y. J. Catal. 2023, 418, 100–109. DOI: https://doi.org/https://doi.org/10.1016/j.jcat.2023.01.008.
Huo, S.; Wang, Q.; Zuo, W. Dalton Trans. 2020, 49, 7959–7967. DOI: https://doi.org/10.1039/D0DT01204A.
Bolitho, E. M.; Worby, N. G.; Coverdale, J. P. C.; Wolny, J. A.; Schünemann, V.; Sadler, P. J. Organometallics. 2021, 40, 3012–3023. DOI: https://doi.org/10.1021/acs.organomet.1c00358.
Wang, W.; Yang, X. Chem. Commun. 2019, 55, 9633–9636. DOI: https://doi.org/10.1039/C9CC04760C.
Zweifel, T.; Naubron, J.-V.; Büttner, T.; Ott, T.; Grützmacher, H. Angew. Chem. Int. Ed. 2008, 47, 3245–3249. DOI: https://doi.org/https://doi.org/10.1002/anie.200704685.
Dubey, A.; Khaskin, E. ACS Catal. 2016, 6, 3998–4002. DOI: https://doi.org/10.1021/acscatal.6b00827.
Weingart, P.; Thiel, W. R. ChemCatChem 2018, 10, 4844–4848. DOI: https://doi.org/https://doi.org/10.1002/cctc.201801334.
Gong, D.; Kong, D.; Xu, N.; Hua, Y.; Liu, B.; Xu, Z. Org. Lett. 2022, 24, 7339–7343. DOI: https://doi.org/10.1021/acs.orglett.2c02866.
Li, Y.; Lian, S.; Wang, J.; Gong, D. Asian J. Org. Chem. 2024, 13, e202300496. DOI: https://doi.org/https://doi.org/10.1002/ajoc.202300496.
Beaufils, A.; Melle, P.; Lentz, N.; Albrecht, M. Inorg. Chem. 2024, 63, 2072–2081. DOI: https://doi.org/10.1021/acs.inorgchem.3c03859.
Patil, R. D.; Pratihar, S. ACS Sustain Chem. Eng. 2024, 12, 6206–6219. DOI: https://doi.org/10.1021/acssuschemeng.3c07989.
Ghosh, D.; Rhodes, S.; Hawkins, K.; Winder, D.; Atkinson, A.; Ming, W.; Padgett, C.; Orvis, J.; Aiken, K.; Landge, S. New J. Chem. 2015, 39, 295–303. DOI: https://doi.org/10.1039/C4NJ01411A.
Wu, S.-Y.; Guo, X.-Q.; Zhou, L.-P.; Sun, Q.-F. Inorg. Chem. 2019, 58, 7091–7098. DOI: https://doi.org/10.1021/acs.inorgchem.9b00756.
Samouei, H.; Grushin, V. V. Organometallics. 2013, 32, 4440–4443. DOI: https://doi.org/10.1021/om400461w.
Liu, W.-P.; Yuan, M.-L.; Yang, X.-H.; Li, K.; Xie, J.-H.; Zhou, Q.-L. Chem. Commun. 2015, 51, 6123–6125. DOI: https://doi.org/10.1039/C5CC00479A.
Tejel, C.; Ciriano, M. A.; Passarelli, V. Chemistry – A Eur. J. 2011, 17, 91–95. DOI: https://doi.org/https://doi.org/10.1002/chem.201002921.

Downloads
Additional Files
Published
Issue
Section
License
Copyright (c) 2024 Lucero González-Sebastián, Ricardo Corona Sánchez, Evelyn Vega Sánchez, Atilano Gutiérrez-Carrillo, Mónica A. Rincón-Guevara

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









