Effect of Glutathione on the Stability, Dynamics and Catalysis of Two Different Classes of Glutathione Transferases from Taenia solium
DOI:
https://doi.org/10.29356/jmcs.v69i1.2305Keywords:
Glutathione transferases, Taenia solium, molecular dynamics simulations, sigma class GSTAbstract
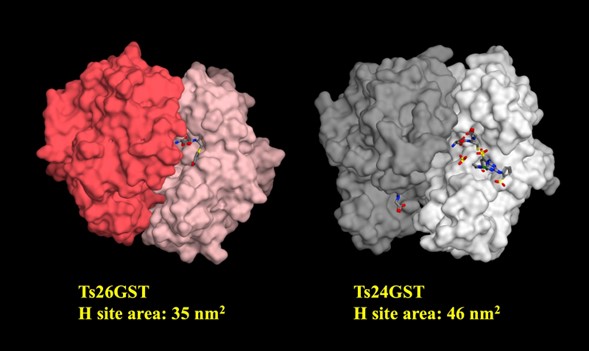
In this work we compare the effect of glutathione (GSH) on the stability and dynamics of two different classes of glutathione transferases from Taenia solium, Ts26GST (a/m class) and Ts24GST (s class). The purpose was to explore why Ts24GST has low catalytic activity for the conjugation of glutathione (GSH) to hydrophobic substrates such as 1-chloro-2,4-dinitrobenzene (CDNB) compared to the very active Ts26GST but can instead use GSH to isomerize prostaglandin H2 to prostaglandin D2 by reducing its peroxide bond, a reaction described just for s class of cytosolic GSTs. Using our recently deposited structure of Ts24GST in the Protein Data Bank, and a previous model for Ts26GST, we determined by molecular dynamics simulations that the presence of GSH decreased the number of intramolecular hydrogen bonds of Ts24GST and increased its radius of gyration, while in Ts26GST the effect was to increase its number of intramolecular hydrogen bonds without significantly changing its radius of gyration. Consistent with this, the experimental thermal stability of Ts26GST increased markedly while that of Ts24GST decreased in the presence of GSH, as determined by intrinsic fluorescence measurements. On the other hand, the binding site for the hydrophobic substrate (H site) of Ts24GST is wider than the H site of Ts26GST, with a 31 % greater solvent-accessible surface area.
Resumen. En este trabajo comparamos el efecto del glutatión (GSH) sobre la estabilidad y la dinámica de dos clases diferentes de glutatión transferasas de Taenia solium, la Ts26GST (clase a/m) y la Ts24GST (clase s). El propósito era explorar por qué la Ts24GST tiene una baja actividad catalítica para la conjugación del glutatión (GSH) con sustratos hidrofóbicos como el 1-cloro-2,4-dinitrobenceno (CDNB) en comparación con la muy activa Ts26GST, pero en cambio puede usar GSH para isomerizar la prostaglandina H2 a prostaglandina D2 reduciendo su enlace peróxido, una reacción descrita solo para las GST citosólicas de clase s. Utilizando nuestra estructura recientemente depositada de la Ts24GST en el Protein Data Bank, y un modelo previo para la Ts26GST, determinamos mediante simulaciones de dinámica molecular que la presencia de GSH disminuyó el número de enlaces de hidrógeno intramoleculares de la Ts24GST y aumentó su radio de giro, mientras que en la Ts26GST el efecto fue aumentar su número de enlaces de hidrógeno intramoleculares sin cambiar significativamente su radio de giro. En consonancia con esto, la estabilidad térmica experimental de la Ts26GST aumentó notablemente mientras que para la Ts24GST disminuyó en presencia de GSH, según lo determinado por mediciones de fluorescencia intrínseca. Por otro lado, el sitio de unión para el sustrato hidrofóbico (sitio H) de la Ts24GST es más ancho que el sitio H de la Ts26GST, con una superficie accesible al disolvente un 31 % mayor.
Downloads
References
Torres-Rivera, A.; Landa, A. Acta Trop. 2008, 105, 99e102. DOI: https://doi.org/10.1016/j.actatropica.2007.08.005
García-Gutiérrez, P.; Zubillaga R. A.; Téllez-Plancarte, A.; Flores-López, R.; Camarillo-Cadena, M.; Landa, A. J. Mol. Graph. Model. 2020, 100, 107707. DOI: https://doi.org/10.1016/j.jmgm.2020.107707
Miranda-Blancas, R.; Rodríguez-Lima, O.; García-Gutiérrez, P. FEBS Open Bio. 2024, 14, 726-739. DOI: https://doi.org/10.1002/2211-5463.13795.
Higgins, L. G.; Hayes, J. D. Drug Metab. Rev. 2011, 43, 92-137. DOI: https://doi.org/10.3109/03602532.2011.567391. PMID: 21495793.
Arbildi, P.; La-Rocca, S.; Kun, A.; Lorenzatto, K. R.; Monteiro, K. M.; Zaha, A.; Mourglia-Ettlin, G.; Ferreira, H. B.; Fernández, V. Acta Trop. 2021, 221, 105991. DOI: https://doi.org/10.1016/j.actatropica.2021.105991.
Madeira, F.; Madhusoodanan, N.; Lee, J.; Eusebi, A.; Niewielska, A.; Tivey, A. R. N.; Lopez, R.; Butcher, S. Nucleic Acids Res. 2024, 52, W521-W525. DOI: https://doi.org/10.1093/nar/gkae241.
Sánchez Pérez L. C.; Zubillaga, R. A.; García-Gutiérrez, P.; Landa, A. Trop. Med. Infect. Dis. 2024, 9, 85. DOI: https://doi.org/10.3390/tropicalmed9040085
Habig, W. H.; Jakoby, W. B. Methods Enzymol. 1981, 77, 398-405. DOI: https://doi.org/10.1016/S0076-6879(81)77053-8
Abraham, M. J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J. C.; Hess, B.; Lindahl, E. SoftwareX. 2015, 1, 19–25. DOI: https://doi.org/10.1016/j.softx.2015.06.001
Dodda, L. S.; Cabeza de Vaca, I.; Tirado-Rives, J.; Jorgensen, W. L. Nucleic Acids Res. 2017, 45, W331-W336. DOI: https://doi.org/10.1093/nar/gkx312
Molecular Operating Environment (MOE). Program for Molecular Modelling. Chemical Computing Group ULC; 910-1010 Sherbooke St. West, Montreal, Quebec, Canada, 2024; https://www.chemcomp.com/
https://server.poissonboltzmann.org/pdb2pqr, accessed in May 2024
Fuentes-Azcatl, R.; Alejandre, J. J. Phys. Chem. B. 2014, 118, 1263-1272. DOI: https://doi.org/10.1021/jp410865y
Pollock, E. L.; Glosli, J. Comput. Phys. Commun. 1996, 85, 93. DOI: https://doi.org/10.1097/00000542-199612000-00039
Parrinello, M.; Rahman, A. J. Appl. Phys. 1981, 52, 7182–7190. DOI: https://doi.org/10.1063/1.328693
Hess, B.; Bekker, H.; Berendsen, H. J. C.; Fraaije, J. G. E. M. J. Comp. Chem. 1997, 18, 1463–1472. DOI: https://doi.org/10.1002/(SICI)1096-987X(199709)18:12<1463::AID-JCC4>3.0.CO;2-H
Fraczkiewicz, R; Braun, W. J. Comp. Chem. 1998, 19, 319-333. DOI: https://doi.org/10.1002/(SICI)1096-987X(199802)19:3<319::AID-JCC6>3.0.CO;2-W
Mohana, K.; Achary, A. Drug Metab. Rev. 2017, 49, 318-337. DOI: https://doi.org/10.1080/03602532.2017.1343343.

Downloads
Published
Issue
Section
License
Copyright (c) 2024 César Sánchez Juárez, Lluvia de Carolina Sánchez Pérez, Rafael A. Zubillaga, Roberto Flores-López, Abraham Landa, Lucía Jiménez, Ricardo Miranda-Blancas, Enrique Rudiño-Piñera, Maria C. Cardona-Echavarría, Ponciano Garcia

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









