Effect of the Linker and Substituents on the Ionic Conductivity of Borate Single-Ion Polymers for Lithium Batteries
DOI:
https://doi.org/10.29356/jmcs.v68i4.2273Keywords:
Lithium-ion, Lithium-ion batteries, polymer electrolytes, ion transport, solid electrolyteAbstract
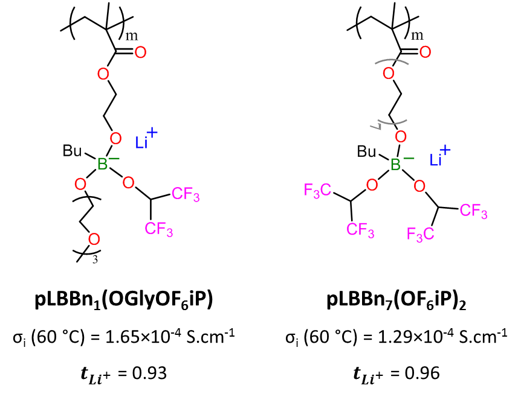
Polymer electrolytes with high ionic conductivity are actively searched for their application as solid electrolytes in lithium batteries. Here, we show new borate single lithium-ion conducting polymers with high ionic conductivity and lithium transference number values. For this purpose, eight new methacrylic lithium borate polymers were synthesized and characterized with varying chemical compositions focusing on the linker between the polymer chain and the pendant borate ionic group and its substituents. The polymers with the optimum ethoxy linker and fluorinated pendant groups show a low Tg value and the highest ionic conductivity value of 1.29×10-4 S.cm-1 at 60 °C. This value is among the highest ionic conductivity reported for a single lithium-ion conducting homopolymer. These polymers show a high lithium transference number (between 0.88 and 0.96) and electrochemical stability close to 4.2 V vs Li+/Li, making them promising candidates for application as solid electrolytes in lithium batteries.
Resumen. Se buscan activamente electrolitos poliméricos con alta conductividad iónica para su aplicación como electrolitos sólidos en baterías de litio. Aquí, mostramos nuevos polímeros conductores de iones de litio de borato simples con valores muy altos de conductividad iónica y número de transferencia de litio. Para ello, se sintetizaron y caracterizaron ocho nuevos polímeros metacrílicos de borato de litio con composiciones químicas variables centradas en el enlazador entre la cadena polimérica y el grupo iónico borato colgante y sus sustituyentes. Los polímeros con el enlazador etoxi óptimo y los grupos colgantes fluorados muestran un valor Tg bajo y un valor superior de conductividad iónica 1,29×10-4 Scm-1 a 60 °C. Este valor es uno de los más altos de conductividad iónica a 60 °C. Este valor es uno de los valores más altos de conductividad iónica a 60 °C. Este valor es uno de los más altos valores de conductividad iónica registrados para un solo homopolímero conductor de iones de litio. Estos polímeros muestran un elevado número de transferencia de litio (entre 0.88 y 0.96), y una estabilidad electroquímica cercana a 4.2 V vs Li+/Li que los convierten en candidatos prometedores para su aplicación como electrolitos sólidos en baterías de litio.
Downloads
References
Nurul, S.; Mohd, A.; Tajuddin, N. A. International Journal of Electrochemical Science. 2021, 16, 1–15. DOI: https://doi.org/10.20964/2021.10.53. DOI: https://doi.org/10.20964/2021.10.53
Zhang, H.; Li, C.; Piszcz, M.; Coya, E.; Rojo, T.; Rodriguez-Martinez, L. M.; Armand, M.; Zhou, Z. Chem Soc Rev. 2017, 3, 797–815. DOI: https://doi.org/10.1039/c6cs00491a. DOI: https://doi.org/10.1039/C6CS00491A
Zhu, J.; Zhang, Z.; Zhao, S.; Westover, A. S.; Belharouak, I.; Cao, P. F. Adv Energy Mater. 2021, 14, 1–18. DOI: https://doi.org/10.1002/aenm.202003836. DOI: https://doi.org/10.1002/aenm.202003836
Mindemark, J.; Lacey, M. J.; Bowden, T.; Brandell, D. Prog. Polym. Sci. 2018, 81, 114–143. DOI: https://doi.org/10.1016/j.progpolymsci.2017.12.004. DOI: https://doi.org/10.1016/j.progpolymsci.2017.12.004
Xue, Z.; He, D.; Xie, X. J. Mater. Chem. A Mater. 2015, 38, 19218–19253. DOI: https://doi.org/10.1039/c5ta03471j. DOI: https://doi.org/10.1039/C5TA03471J
Suo, L.; Zheng, F.; Hu, Y. S.; Chen, L. Chinese Physics B. 2015, 1, 0–4. DOI: https://doi.org/10.1088/1674-1056/25/1/016101. DOI: https://doi.org/10.1088/1674-1056/25/1/016101
Jiang, Y.; Yan, X.; Ma, Z.; Mei, P.; Xiao, W.; You, Q.; Zhang, Y. Polymers. 2018, 4, 1–13. DOI: https://doi.org/10.3390/polym10111237. DOI: https://doi.org/10.3390/polym10111237
Croce, F.; Appetecchi, G. B.; Persi, L.; Scrosati, B. Nature. 1998, 394, 456–458. DOI: https://doi.org/10.1038/28818. DOI: https://doi.org/10.1038/28818
Guzmán-González, G.; Avila-Paredes, H. J.; Santos-Mendoza, I. Journal of Solid-State Electrochemistry. 2023. DOI: https://doi.org/10.1007/s10008-023-05563-1. DOI: https://doi.org/10.1007/s10008-023-05563-1
Porcarelli, L.; Shaplov, A. S.; Bella, F.; Nair, J. R.; Mecerreyes, D.; Gerbaldi, C. ACS Energy Lett. 2016, 4, 678–682. DOI: https://doi.org/10.1021/acsenergylett.6b00216. DOI: https://doi.org/10.1021/acsenergylett.6b00216
Shan, X.; Zhao, S.; Ma, M.; Pan, Y.; Xiao, Z.; Li, B.; Sokolov, A. P.; Tian, M.; Yang, H.; Cao, P. F. ACS Appl. Mater Interfaces. 2022, 14, 56110−56119. https://doi.org/10.1021/acsami.2c17547. DOI: https://doi.org/10.1021/acsami.2c17547
Guzman Gonzalez, G. J. Mex. Chem. Soc. 2023, 4, 602–620. DOI: https://doi.org/10.29356/jmcs.v67i4.1959. DOI: https://doi.org/10.29356/jmcs.v67i4.1959
Porcarelli, L.; Vlasov, P. S.; Ponkratov, D. O.; Lozinskaya, E. I.; Antonov, D. Y.; Nair, J. R.; Gerbaldi, C.; Mecerreyes, D.; Shaplov, A. S. Eur. Polym. J. 2018, 107, 218–228. DOI: https://doi.org/10.1016/j.eurpolymj.2018.08.014. DOI: https://doi.org/10.1016/j.eurpolymj.2018.08.014
Zygadła-Monikowska, E.; Florjańczyk, Z.; Ostrowska, J.; Bołtromiuk, P.; Frydrych, J.; Sadurski, W.; Langwald, N. Electrochim. Acta. 2011, 1, 66–73. DOI: https://doi.org/10.1016/j.electacta.2011.07.120. DOI: https://doi.org/10.1016/j.electacta.2011.07.120
Guzmán‐González, G.; Alvarez‐Tirado, M.; Olmedo‐Martínez, J. L.; Picchio, M. L.; Casado, N.; Forsyth, M.; Mecerreyes, D. Adv. Energy Mater. 2023, 1, 2202974. DOI: https://doi.org/10.1002/aenm.202202974. DOI: https://doi.org/10.1002/aenm.202202974
Porcarelli, L.; Shaplov, A. S.; Salsamendi, M.; Nair, J. R.; Vygodskii, Y. S.; Mecerreyes, D.; Gerbaldi, C. ACS Appl Mater Interfaces. 2016, 16, 10350–10359. DOI: https://doi.org/10.1021/acsami.6b01973. DOI: https://doi.org/10.1021/acsami.6b01973
Shaplov, A. S.; Vlasov, P. S.; Armand, M.; Lozinskaya, E. I.; Ponkratov, D. O.; Malyshkina, I. A.; Vidal, F.; Okatova, O. V.; Pavlov, G. M.; Wandrey, C.; Godovikov, I. A.; Vygodskii, Y. S. Polym Chem. 2011, 11, 2609–2618. DOI: https://doi.org/10.1039/c1py00282a. DOI: https://doi.org/10.1039/c1py00282a
Meziane, R.; Bonnet, J. P.; Courty, M.; Djellab, K.; Armand, M. Electrochim Acta. 2011, 1, 14–19. DOI: https://doi.org/10.1016/j.electacta.2011.03.074. DOI: https://doi.org/10.1016/j.electacta.2011.03.074
Ma, Q.; Zhang, H.; Zhou, C.; Zheng, L.; Cheng, P.; Nie, J.; Feng, W.; Hu, Y. S.; Li, H.; Huang, X.; Chen, L.; Armand, M.; Zhou, Z. Angewandte Chemie - International Edition. 2016, 7, 2521–2525. DOI: https://doi.org/10.1002/anie.201509299. DOI: https://doi.org/10.1002/anie.201509299
Zhu, Y. S.; Wang, X. J.; Hou, Y. Y.; Gao, X. W.; Liu, L. L.; Wu, Y. P.; Shimizu, M. Electrochim Acta. 2013, 87, 113–118. DOI: https://doi.org/10.1016/j.electacta.2012.08.114. DOI: https://doi.org/10.1016/j.electacta.2012.08.114
Ponkratov, D. O.; Lozinskaya, E. I.; Shaplov, A. S.; Khanin, D. A.; Afanasyev, E. S.; Takazova, R. U.; Vygodskii, Y. S. Doklady Chemistry. 2022, 2, 29–36. DOI: https://doi.org/10.1134/S0012500822020021. DOI: https://doi.org/10.1134/S0012500822020021
Rolland, J.; Brassinne, J.; Bourgeois, J. P.; Poggi, E.; Vlad, A.; Gohy, J. F. J Mater Chem. A Mater. 2014, 30, 11839–11846. DOI: https://doi.org/10.1039/c4ta02327g. DOI: https://doi.org/10.1039/C4TA02327G
Rolland, J.; Poggi, E.; Vlad, A.; Gohy, J. F. Polymer. 2015, 68, 344–352. DOI: https://doi.org/10.1016/j.polymer.2015.04.056. DOI: https://doi.org/10.1016/j.polymer.2015.04.056
Olmedo-Martínez, J. L.; Porcarelli, L.; Alegría, Á.; Mecerreyes, D.; Müller, A. J. Macromolecules. 2020, 11, 4442–4453. DOI: https://doi.org/10.1021/acs.macromol.0c00703. DOI: https://doi.org/10.1021/acs.macromol.0c00703
Meabe, L.; Goujon, N.; Li, C.; Armand, M.; Forsyth, M.; Mecerreyes, D. Batter Supercaps. 2020, 1, 68–75. DOI: https://doi.org/10.1002/batt.201900119. DOI: https://doi.org/10.1002/batt.201900119
Guzmán-González, G.; Vauthier, S.; Alvarez-Tirado, M.; Cotte, S.; Castro, L.; Guéguen, A.; Casado, N.; Mecerreyes, D. Angewandte Chemie - International Edition. 2021, 7, 1–5. DOI: https://doi.org/10.1002/anie.202114024. DOI: https://doi.org/10.1002/anie.202114024
Zygadło-Monikowska, E.; Florjańczyk, Z.; Słuzewska, K.; Ostrowska, J.; Langwald, N.; Tomaszewska, A. J. Power Sources. 2010, 18, 6055–6061. DOI: https://doi.org/10.1016/j.jpowsour.2009.12.097. DOI: https://doi.org/10.1016/j.jpowsour.2009.12.097
Meabe, L.; Huynh, T. V.; Lago, N.; Sardon, H.; Li, C.; O’Dell, L. A.; Armand, M.; Forsyth, M.; Mecerreyes, D. Electrochim Acta. 2018, 264, 367–375. DOI: https://doi.org/10.1016/j.electacta.2018.01.101. DOI: https://doi.org/10.1016/j.electacta.2018.01.101
Qian, X.; Gu, N.; Cheng, Z.; Yang, X.; Wang, E.; Dong, S. Journal of Solid-State Electrochemistry. 2001, 1, 8–15. DOI: https://doi.org/10.1007/s100080000190. DOI: https://doi.org/10.1007/s100080000190
Menzinger, M.; Wolfgang, R. Angewandte Chemie International Edition in English. 1969, 6, 438–444. DOI: https://doi.org/10.1002/anie.196904381. DOI: https://doi.org/10.1002/anie.196904381
Soydan, A. M.; Bozkurt, A. Ionics. 2018, 5, 1399–1405. DOI: https://doi.org/10.1007/s11581-017-2286-4. DOI: https://doi.org/10.1007/s11581-017-2286-4
Evans, J.; Vincent, C. A.; Bruce, P. G. Polymer. 1987, 13, 2324–2328. DOI: https://doi.org/10.1016/0032-3861(87)90394-6 . DOI: https://doi.org/10.1016/0032-3861(87)90394-6
Zugmann, S.; Fleischmann, M.; Amereller, M.; Gschwind, R. M.; Wiemhöfer, H. D.; Gores, H. J. Electrochim Acta, 2011, 11, 3926–3933. DOI: https://doi.org/10.1016/j.electacta.2011.02.025. DOI: https://doi.org/10.1016/j.electacta.2011.02.025
Strauss, E.; Menkin, S.; Golodnitsky, D. Journal of Solid State Electrochemistry. 2017, 7, 1879–1905. DOI: https://doi.org/10.1007/s10008-017-3638-8. DOI: https://doi.org/10.1007/s10008-017-3638-8
Hamaide, T.; Le Deore, C. Polymer. 1993, 5, 1038–1046. DOI: https://doi.org/10.1016/0032-3861(93)90227-2. DOI: https://doi.org/10.1016/0032-3861(93)90227-2
Zygadło-Monikowska, E.; Florjańczyk, Z.; Tomaszewska, A.; Pawlicka, M.; Langwald, N.; Kovarsky, R.; Mazor, H.; Golodnitsky, D.; Peled, E. Electrochim Acta. 2007, 4, 1481–1489. DOI: https://doi.org/10.1016/j.electacta.2007.02.046. DOI: https://doi.org/10.1016/j.electacta.2007.02.046
Guzmán-González, G.; Ávila-Paredes, H. J.; Rivera, E.; González, I. ACS Appl Mater Interfaces. 2018, 36, 30247–30256. DOI: https://doi.org/10.1021/acsami.8b02519. DOI: https://doi.org/10.1021/acsami.8b02519
Guzmán-González, G.; Ramos-Sánchez, G.; Camacho-Forero, L. E.; González, I. Journal of Physical Chemistry C. 2019, 29, 17686–17694. DOI: https://doi.org/10.1021/acs.jpcc.9b02945. DOI: https://doi.org/10.1021/acs.jpcc.9b02945
G. Guzmán, D. Nava, J. Vasquez-Arenas, J. Cardoso, J. A.-Ramirez. Solid State Ionics. 2019, 5, 55.
Nava, D. P.; Guzmán, G.; Vazquez-Arenas, J.; Cardoso, J.; Gomez, B.; Gonzalez, I. Solid State Ionic. 2016, 290, 98–107. DOI: https://doi.org/10.1016/j.ssi.2016.03.020. DOI: https://doi.org/10.1016/j.ssi.2016.03.020
Downloads
Additional Files
Published
Issue
Section
License
Copyright (c) 2024 Soline Vauthier, Stéphane Cotte, Laurent Castro, Aurélie Guéguen, Nerea Casado, David Mecerreyes, Gregorio Guzman Gonzalez

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









