Pyrone Biomonitored Synthesis
DOI:
https://doi.org/10.29356/jmcs.v69i2.2213Keywords:
Yangonin, styrylpyrone, antifungal, antibacterial, antimicrobial, pyroneAbstract
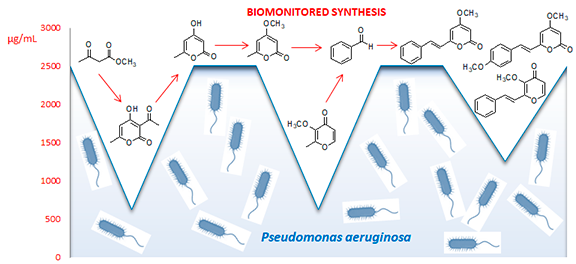
Abstract. Here we report the first biomonitored synthesis of pyrones in the search for molecules with antimicrobial action against pathogenic bacteria and fungi. Pyrones were synthesized from methyl acetoacetate pyrone rings followed by deacetylation, methylation, and aldol condensation reactions to obtain styrylpyrones with yields between 39 and 93 %. The compounds were characterized based on the interpretation of their UV, IR, MS and 1H and 13C NMR spectra. The reagents and products used in the first step of the reaction exhibited antimicrobial activity against the six microorganisms tested, except for methyl acetoacetate and benzaldehyde, which were inactive against Klebsiella pneumoniae bacteria. The results obtained contribute significantly to the knowledge of the antimicrobial potential of pyrones, considering that pyrone rings are widely used as building blocks in the synthesis of bioactive molecules. This is also the first report of antimicrobial activity for synthesized styrylpyrone.
Resumen. Aquí informamos la primera síntesis biomonitoreada de pironas en la búsqueda de moléculas con acción antimicrobiana contra bacterias y hongos patogénicos. Las pironas se sintetizaron a partir de anillos de pirona de acetoacetato de metilo seguido de reacciones de desacetilación, metilación y condensación aldólica para obtener estirilpironas con rendimientos entre 39 y 93 %. Los compuestos se caracterizaron basándose en la interpretación de sus espectros UV, IR, MS y RMN 1H y 13C. Los reactivos y productos utilizados en el primer paso de la reacción mostraron actividad antimicrobiana contra los seis microorganismos probados, excepto el acetoacetato de metilo y el benzaldehído, que fueron inactivos contra la bacteria Klebsiella pneumoniae. Los resultados obtenidos contribuyen significativamente al conocimiento del potencial antimicrobiano de las pironas, considerando que los anillos de pirona son ampliamente utilizados como componentes básicos en la síntesis de moléculas bioactivas. Este es también el primer informe sobre la actividad antimicrobiana de la estirilpirona sintetizada.
Downloads
References
Kang, L.; Jing, W.; Liu, Q.; Liu, J.; Liu, M. J. Infect. Public Health. 2022, 15, 870–876. DOI: https://doi.org/10.1016/j.jiph.2022.06.016. DOI: https://doi.org/10.1016/j.jiph.2022.06.016
Carvalho, I.; Silva, N.; Carrola, J.; Silva, V.; Currie, C.; Igrejas, G.; Poeta, P., in: Antibiotic Drug Resistance; Eds.; Wiley, 2019; Vol. 1, 239–259. DOI: https://doi.org/10.1002/9781119282549.ch11
Jaramillo, M. A.; Callejas, R., in: Piper: A Model Genus for Studies of Phytochemistry, Ecology, and
Evolution, Ed., Springer US: Boston, 2004; 179–198.
Costa-Lotufo, L. V.; Montenegro, R. C.; Alves, A. P. N. N.; Madeira, S. V. F.; Pessoa, C.; Moraes, M.
E. A. D.; Moraes, M. O. D. Rev. Virtual Quím. 2010, 2, 47–58. DOI: https://doi.org/10.5935/1984-6835.20100006. DOI: https://doi.org/10.5935/1984-6835.20100006
Koehn, F. E.; Carter, G. T. Nat. Rev. Drug Discov. 2005, 4, 206–220. DOI: https://doi.org/10.1038/nrd1657. DOI: https://doi.org/10.1038/nrd1657
Newman, D. J.; Cragg, G. M. J. Nat. Prod. 2012, 75, 311-335. DOI:https://doi.org/10.1021/np200906s. DOI: https://doi.org/10.1021/np200906s
Newman, D. J.; Cragg, G. M. J. Nat. Prod. 2016, 79, 629–661. DOI: https://doi.org/10.1021/acs.jnatprod.5b01055. DOI: https://doi.org/10.1021/acs.jnatprod.5b01055
Righetti, G. I. C.; Tentori, F.; Brenna, E.; Gambarotti, C. React. Chem. Eng. 2023, 8, 199–204. DOI: https://doi.org/10.1039/D2RE00312K. DOI: https://doi.org/10.1039/D2RE00312K
Yi, D.; Agarwal, V. ACS Chem. Biol. 2023, 18, 1060–1065. DOI: https://doi.org/10.1021/acschembio.3c00081. DOI: https://doi.org/10.1021/acschembio.3c00081
Luo, C.; Xu, X.; Xu, J.; Chen, X. Org. Biomol. Chem. 2022, 20, 9298–9301. DOI:
https://doi.org/10.1039/D2OB01859D. DOI: https://doi.org/10.1039/D2OB01859D
Hu, C.; Jiang, L.; Tang, L.; Zhang, M.; Sheng, R. Bioorg. Med. Chem. 2021, 44, 116306. DOI: https://doi.org/10.1016/j.bmc.2021.116306. DOI: https://doi.org/10.1016/j.bmc.2021.116306
Obi, G.; Chukwujekwu, J. C.; Van Heerden, F. R. Synth. Commun. 2020, 50, 726–734. DOI: https://doi.org/10.1080/00397911.2020.1718710. DOI: https://doi.org/10.1080/00397911.2020.1718710
Xue, L.-W.; Han, Y.-J.; Luo, X.-Q. Acta Chim. Slov. 2019, 66, 622–628. DOI: https://doi.org/10.17344/acsi.2019.5039. DOI: https://doi.org/10.17344/acsi.2019.5039
Singh, K. S. Curr. Org. Chem. 2020, 24, 354–401. DOI:
https://doi.org/10.2174/1385272824666200217101400. DOI: https://doi.org/10.2174/1385272824666200217101400
Da Silva, A.; M. Da Silva, J.; V. Almeida, A.; S. Ramos, C. Nat. Prod. J. 2016, 6, 313–317. DOI: https://doi.org/10.26850/1678-4618eqj.v42.1.2017. DOI: https://doi.org/10.2174/2210315506666160916152524
Freitas Filho, J. R.; de Holanda, L. E. G.; Ramos, C. S. J. Mex. Chem. Soc. 2023, 67, 163-171. DOI: https://doi.org/ 10.29356/jmcs.v67i2.1866. DOI: https://doi.org/10.29356/jmcs.v67i2.1866
Nagawade, R. R.; Khanna, V. V.; Bhagwat, S. S.; Shinde, D. B. Eur. J. Med. Chem. 2005, 40, 1325– 1330. DOI: https://doi.org/10.1016/j.ejmech.2005.05.012. DOI: https://doi.org/10.1016/j.ejmech.2005.05.012
Filipponi, P.; Baxendale, I. R. Eur. J. Org. Chem. 2016, 2016, 2000–2012. DOI: https://doi.org/10.1021/acs.oprd.5b00331. DOI: https://doi.org/10.1002/ejoc.201600222
Kraus, G. A.; Wanninayake, U. K. Tetrahedron Lett. 2015, 56, 7112–7114. DOI: http://dx.doi.org/10.1016/j.tetlet.2016.02.043. DOI: https://doi.org/10.1016/j.tetlet.2015.11.021
Van, T.; Xuan, T.; Minh, T.; Quan, N. Molecules. 2018, 23, 1–13. DOI: https://doi.org/10.3390/molecules23081907. DOI: https://doi.org/10.3390/molecules23081907
Kumagai, M.; Mishima, T.; Watanabe, A.; Harada, T.; Yoshida, I.; Fujita, K.; Watai, M.; Tawata, S.; Nishikawa, K.; Morimoto, Y. Biosci. Biotechnol. Biochem. 2016, 80, 1425–1432. DOI: https://doi.org/10.1080/09168451.2016.1153959. DOI: https://doi.org/10.1080/09168451.2016.1153959
Soldi, C.; Moro, A. V.; Pizzolatti, M. G.; Correia, C. R. D. Eur. J. Org. Chem. 2012, 2012, 3607– 3616. DOI: https://doi.org/10.1002/ejoc.201200308. DOI: https://doi.org/10.1002/ejoc.201200308
Upadhyay, A.; Chompoo, J.; Kishimoto, W.; Makise, T.; Tawata, S. J. Agric. Food Chem. 2011, 59, 2857–2862. DOI: https://doi.org/10.1021/jf104813k. DOI: https://doi.org/10.1021/jf104813k
Manda, B.; Prasad, A.; Thatikonda, N.; Lacerda Jr., V.; Barbosa, L.; Santos, H.; Romão, W.; Pavan, F.; Ribeiro, C.; Dos Santos, E.; et al. J. Braz. Chem. Soc. 2018, 29, 639–648. DOI: https://dx.doi.org/10.21577/0103-5053.20170178. DOI: https://doi.org/10.21577/0103-5053.20170178
De Paiva, R.; Da Silva, J.; Moreira, H.; Pinto, O.; Camargo, L.; Naves, P.; Camargo, A.; Ribeiro, L.; Ramos, L. J. Braz. Chem. Soc. 2019, 30, 164–172. DOI: https://doi.org/10.21577/0103-5053.20180158. DOI: https://doi.org/10.21577/0103-5053.20180158
Nechak, R.; Achouche Bouzroura, S.; Benmalek, Y.; Boufroua, N.; Nedjar Kolli, B.; Poulain Martini, S.; Duñach, E. Synth. Commun. 2019, 49, 1895–1905. DOI: https://doi.org/10.1080/00397911.2019.1606918. DOI: https://doi.org/10.1080/00397911.2019.1606918
Fadda, A. A.; Amine, M. S.; Arief, M. M. H.; Farahat, E. Kh. Pharmacologia. 2014, 5, 1–11. https://scialert.net/abstract/?doi=pharmacologia.2014.1.11. DOI: https://doi.org/10.5567/pharmacologia.2014.1.11
Baldwin, A. G.; Bevan, J.; Brough, D.; Ledder, R.; Freeman, S. Med. Chem. Res. 2018, 27, 884–889. DOI: https://doi.org/10.1007/s00044-017-2110-8. DOI: https://doi.org/10.1007/s00044-017-2110-8
Zheng, Y. Y.; Liang, Z. Y.; Shen, N. X.; Liu, W. L.; Zhou, X. J.; Fu, X. M.;Wang, C. Y. Mar. Drugs.
, 17, 322. DOI: https://doi.org/10.3390/md17060322. DOI: https://doi.org/10.3390/md17060322
He, Y.; Tian, J.; Chen, X., Sun, W.; Zhu, H.; Li, Q.; Zhang, Y. Sci. Rep. 2016, 6, 24291. DOI:
https://doi.org/10.1038/srep24291. DOI: https://doi.org/10.1038/srep24291
Chaurasiya, N. D.; León, F.; Ding, Y.; Gómez-Betancur, I.; Benjumea, D.; Walker, L. A.; Cutler, S. J.; Tekwani, B. L. Evid. Based Complement. Alternat. Med. 2017, 2017, 1–10. DOI: https://doi.org/10.1155/2017/4018724. DOI: https://doi.org/10.1155/2017/4018724
Chou, T.-W.; Feng, J.-H.; Huang, C.-C.; Cheng, Y.-W.; Chien, S.-C.; Wang, S.-Y.; Shyur, L.-F. PLoS
ONE. 2013, 8, e77626. DOI: https://doi.org/10.1371/journal.pone.0077626. DOI: https://doi.org/10.1371/journal.pone.0077626
Dong, R.; Wang, X.; Wang, L.; Wang, C.; Huang, K.; Fu, T.; Liu, K.; Wu, J.; Sun, H.; Meng, Q. Eur. J. Parmachol. 2021, 890, 173653. DOI: https://doi.org/10.1016/j.ejphar.2020.173653. DOI: https://doi.org/10.1016/j.ejphar.2020.173653
Downloads
Additional Files
Published
Issue
Section
License
Copyright (c) 2025 Clécio Souza Ramos, Marcílio Wagner Fontes Silva

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









