Fabrication of a Reactive Functionalized Microfilm with Aromatic Amines Applied to the Growth of Langerhans Cells
DOI:
https://doi.org/10.29356/jmcs.v68i1.2081Keywords:
Ultrathin films, azlactone, β-cells, aromatic amineAbstract
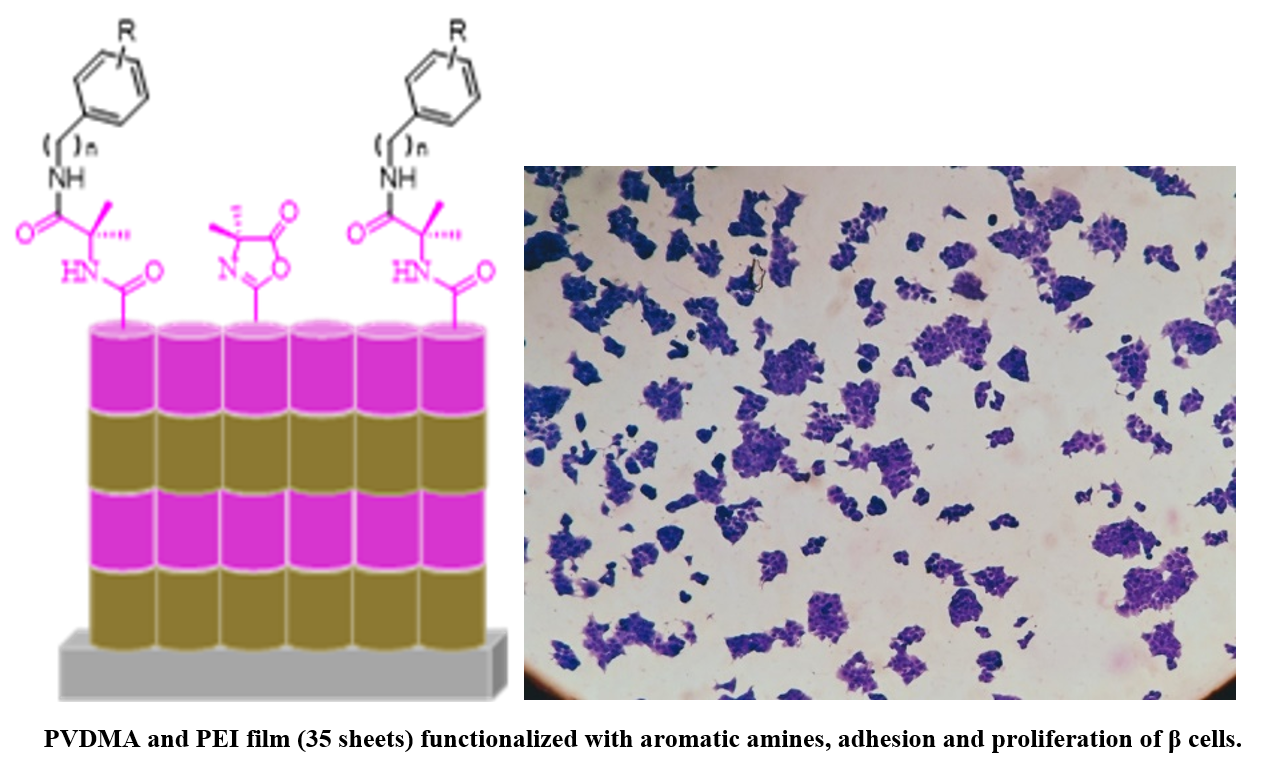
This study reports the synthesis of ultrathin polymeric films through layer-by-layer deposition and covalent cross-linking of poly(2-vinyl-4,4'-dimethylazlactone) and branched poly (ethylene imine) (PEI) which were functionalized with aromatic amines that encompass anilines. To assess the effect of these aromatics molecules on the adhesion and proliferation of Langerhans β-cells, we prepared 35 bilayers of unfunctionalized and functionalized films with aromatic amines, which were characterized in terms of their physical, chemical, and biological properties by a battery of experimental techniques including 1H and 13C, NMR, mass spectrometry, attenuated total reflectance Fourier transform infrared spectroscopy, field emission scanning electron microscopy and cell adhesion and staining. The films were nanometric, transparent, resistant to manipulation, chemically reactive, and highly cytocompatible. We demonstrated that films functionalized with aromatic molecules support the attachment and growth of in vitro Langerhans β-cells. This study provides the basis for a general approach to designing and functionalizing ultrathin films that promote cell growth on surfaces of interest for investigation in cell biology studies and a broad range of other biomedical applications.
Resumen. En este estudio se describe la síntesis de películas poliméricas ultrafinas mediante la técnica de capa por capa y la reticulación covalente de poli(2-vinil-4,4'-dimetilazlactona) y poli etilenimina (PEI) ramificado y, se funcionalizaron con aminas aromáticas que engloba las anilinas. Para evaluar el efecto de estas moléculas aromáticas en la adhesión y proliferación de las células β de Langerhans, se prepararon películas de 35 bicapas y se funcionalizaron con aminas aromáticas; se caracterizaron en términos de sus propiedades físicas, químicas y biológicas mediante una serie de técnicas experimentales que incluyeron 1H y 13C, RMN, espectrometría de masas, espectroscopia de infrarrojo por transformada de Fourier, microscopía electrónica de barrido por emisión de campo y tinción celular. En general, las películas fueron nanométricas, transparentes, resistentes a la manipulación, químicamente reactivas y altamente citocompatibles. Se demostró, además, que las películas funcionalizadas con moléculas aromáticas favorecen la adhesión y el crecimiento de células β in vitro. Este estudio establece las bases de un enfoque general para diseñar y funcionalizar películas ultrafinas, que promuevan el crecimiento celular en superficies de interés, para la investigación en estudios de biología celular y una gama amplia de aplicaciones biomédicas potenciales.
Downloads
References
Hudish, L. I.; Reusch, J. E. B.; Sussel, L. J. Clin. Invest. 2019, 129, 4001–4008. DOI: https://doi.org/10.1172/JCI129188.
Smith, G. I.; Mittendorfer, B.; Klein, S. J. Clin. Invest. 2019, 129 , 3978–3989. DOI: https://doi.org/10.1172/JCI129186.
Reaven, G. M. Annu. Rev. 2003, 44, 121–131. DOI: https://doi.org/10.1146/ANNUREV.ME.44.020193.001005.
Reaven, G. M. Diabetes 1988, 37, 1595–1607. DOI: https://doi.org/10.2337/DIAB.37.12.1595.
O’Neill, S.; O’Driscoll, L. Obes. Rev. 2015, 16 , 1–12. DOI: https://doi.org/10.1111/OBR.12229.
Lorenzo, C.; Okoloise, M.; Williams, K.; Stern, M. P.; Haffner, S. M. Diabetes Care- 2003, 26, 3153–3159. DOI: https://doi.org/10.2337/DIACARE.26.11.3153.
Anuradha, R.; Saraswati, M.; Kumar, K. G.; Rani, S. H. DNA Cell Biol. 2014, 33, 743–748. DOI: https://doi.org/10.1089/DNA.2014.2352.
Dotta, F.; Fondelli, C.; Di Mario, U. Acta Biomed. 2005, 76 Suppl 3, 14–18.
Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Diabetes Care. 2004, 27, 1047–1053. DOI: https://doi.org/10.2337/DIACARE.27.5.1047.
Pérez-Bravo, F.; Carrasco, E.; Gutierrez-López, M. D.; Martínez, M. T.; Lopez, G.; García De Los Rios, M. J. Mol. Med. (Berl). 1996, 74, 105–109. DOI: https://doi.org/10.1007/BF00196786.
Krijnen, P. A. J.; Simsek, S.; Niessen, H. W. M. Apoptosis. 2009, 14, 1387. DOI: https://doi.org/10.1007/S10495-009-0419-6.
Martinez, S. C.; Tanabe, K.; Cras-Méneur, C.; Abumrad, N. A.; Bernal-Mizrachi, E.; Permutt, M. A. Diabetes. 2008, 57, 846–859. DOI: https://doi.org/10.2337/DB07-0595.
Randle, P. J.; Garland, P. B.; Hales, C. N.; Newsholme, E. A. Lancet. 1963, 1, 785–789. DOI: https://doi.org/10.1016/S0140-6736(63)91500-9.
Newsholme, P.; Keane, D.; Welters, H. J.; Morgan, N. G. Clin. Sci. (Lond). 2007, 112, 27–42. DOI: https://doi.org/10.1042/CS20060115.
Acosta-Montaño, P.; Rodríguez-Velázquez, E.; Ibarra-López, E.; Frayde-Gómez, H.; Mas-Oliva, J.; Delgado-Coello, B.; Rivero, I. A.; Alatorre-Meda, M.; Aguilera, J.; Guevara-Olaya, L.; García-González, V. Cells. 2019, 8. DOI: https://doi.org/10.3390/CELLS8080884.
Lewis, G. F.; Carpentier, A.; Adeli, K.; Giacca, A. Endocr. Rev. 2002, 23, 201–229. DOI: https://doi.org/10.1210/EDRV.23.2.0461.
Acosta-Montaño, P.; García-González, V. Nutrients. 2018, 10. DOI: https://doi.org/10.3390/NU10040393.
Heimberg, H.; De Vos, A.; Vandercammen, A.; Van Schaftingen, E.; Pipeleers, D.; Schuit, F. EMBO J. 1993, 12, 2873–2879. DOI: https://doi.org/10.1002/J.1460-2075.1993.TB05949.X.
Gupta, D.; Jetton, T. L.; LaRock, K.; Monga, N.; Satish, B.; Lausier, J.; Peshavaria, M.; Leahy, J. L. J. Biol. Chem. 2017, 292, 12449–12459. DOI: https://doi.org/10.1074/JBC.M117.781047.
Poitout, V.; Amyot, J.; Semache, M.; Zarrouki, B.; Hagman, D.; Fontés, G. Biochim. Biophys. Acta. 2010, 1801, 289–298. DOI: https://doi.org/10.1016/J.BBALIP.2009.08.006.
Donath, M. Y.; Shoelson, S. E. Nat. Rev. Immunol. 2011, 11, 98–107. DOI: https://doi.org/10.1038/NRI2925.
Ito, Y. Biomaterials. 1999, 20, 2333–2342. DOI: https://doi.org/10.1016/S0142-9612(99)00162-3.
Wancura, M. M.; Anex-Ries, Q.; Carroll, A. L.; Paola Garcia, A.; Hindocha, P.; Buck, M. E. J. Polym. Sci. A Polym. Chem. 2017, 55, 3185–3194. DOI: https://doi.org/10.1002/POLA.28664.
Buck, M. E.; Breitbach, A. S.; Belgrade, S. K.; Blackwell, H. E.; Lynn, D. M. Biomacromolecules. 2009, 10, 1564. DOI: https://doi.org/10.1021/BM9001552.
Singhvi, R.; Kumar, A.; Lopez, G. P.; Stephanopoulos, G. N.; Wang, D. I. C.; Whitesides, G. M.; Ingber, D. E. Science. 1994, 264, 696–698. DOI: https://doi.org/10.1126/SCIENCE.8171320.
Ávila-Cossío, M. E.; Rivero, I. A.; García-González, V.; Alatorre-Meda, M.; Rodríguez-Velázquez, E.; Calva-Yáñez, J. C.; Espinoza, K. A.; Pulido-Capiz, Á. ACS Omega. 2020, 5, 5249–5257. DOI: https://doi.org/10.1021/ACSOMEGA.9B04313.
Nadal, A.; Alonso-Magdalena, P.; Soriano, S.; Quesada, I.; Ropero, A. B. Mol. Cell Endocrinol. 2009, 304, 63–68. DOI: https://doi.org/10.1016/J.MCE.2009.02.016.
Decher, G. Science (1979). 1997, 277, 1232–1237. DOI: https://doi.org/10.1126/SCIENCE.277.5330.1232.
Tang, Z.; Wang, Y.; Podsiadlo, P.; Kotov, N. A. Adv.Mater. 2006, 18, 3203–3224. DOI: https://doi.org/10.1002/ADMA.200600113.
Buck, M. E.; Zhang, J.; Lynn, D. M. Adv. Mater. 2007, 19, 3951–3955. DOI: https://doi.org/10.1002/ADMA.200700822.
Kolb, H. C.; Sharpless, K. B. Drug Discov. Today. 2003, 8, 1128–1137. DOI: https://doi.org/10.1016/S1359-6446(03)02933-7.
Mrksich, M. Chem. Soc. Rev. 2000, 29, 267–273. DOI: https://doi.org/10.1039/A705397E.
Guevara-Olaya, L.; Chimal-Vega, B.; Castañeda-Sánchez, C. Y.; López-Cossio, L. Y.; Pulido-Capiz, A.; Galindo-Hernández, O.; Díaz-Molina, R.; Ruiz Esparza-Cisneros, J.; García-González, V. Metabolites. 2022, 12. DOI: https://doi.org/10.3390/METABO12080754.
Martínez-Navarro, I.; Díaz-Molina, R.; Pulido-Capiz, A.; Mas-Oliva, J.; Luna-Reyes, I.; Rodríguez-Velázquez, E.; Rivero, I. A.; Ramos-Ibarra, M. A.; Alatorre-Meda, M.; García-González, V. Biomolecules. 2020, 10, 1–21. DOI: https://doi.org/10.3390/BIOM10091201.
Manna, U.; Raman, N.; Welsh, M. A.; Zayas-Gonzalez, Y. M.; Blackwell, H. E.; Palecek, S. P.; Lynn, D. M. Adv. Funct. Mater. 2016, 26, 3599–3611. DOI: https://doi.org/10.1002/ADFM.201505522.
Broderick, A. H.; Azarin, S. M.; Buck, M. E.; Palecek, S. P.; Lynn, D. M. in: Fabrication of Amine-Reactive Polymer Multilayers on Microwell Cell Culture Arrays: Combining Methods for the Topographic Patterning of Cell Substrates with Approaches to Facile Surface Functionalization. AIChE January 1, 2011, 72–73. https://experts.umn.edu/en/publications/fabrication-of-amine-reactive-polymer-multilayers-on-microwell-ce, accessed June 2023.
Weeks, C. A.; Aden, B.; Zhang, J.; Singh, A.; Hickey, R. D.; Kilbey, S. M.; Nyberg, S. L.; Janorkar, A. V. J. Biomed. Mater. Res. A. 2017, 105, 377–388. DOI: https://doi.org/10.1002/JBM.A.35910.
Li, Y.; Wang, X.; Sun, J. Chem. Soc. Rev. 2012, 41, 5998–6009. DOI: https://doi.org/10.1039/C2CS35107B

Downloads
Additional Files
Published
Issue
Section
License
Copyright (c) 2023 Ignacio A. Rivero Espejel, martha avila, victor García-González

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









