Biosynthesis of Silver Nanoparticles Mediated by Lippia graveolens Aqueous Extract
DOI:
https://doi.org/10.29356/jmcs.v68i3.2070Keywords:
Silver nanoparticles, Mexican oregano, antimicrobial activity, green synthesisAbstract
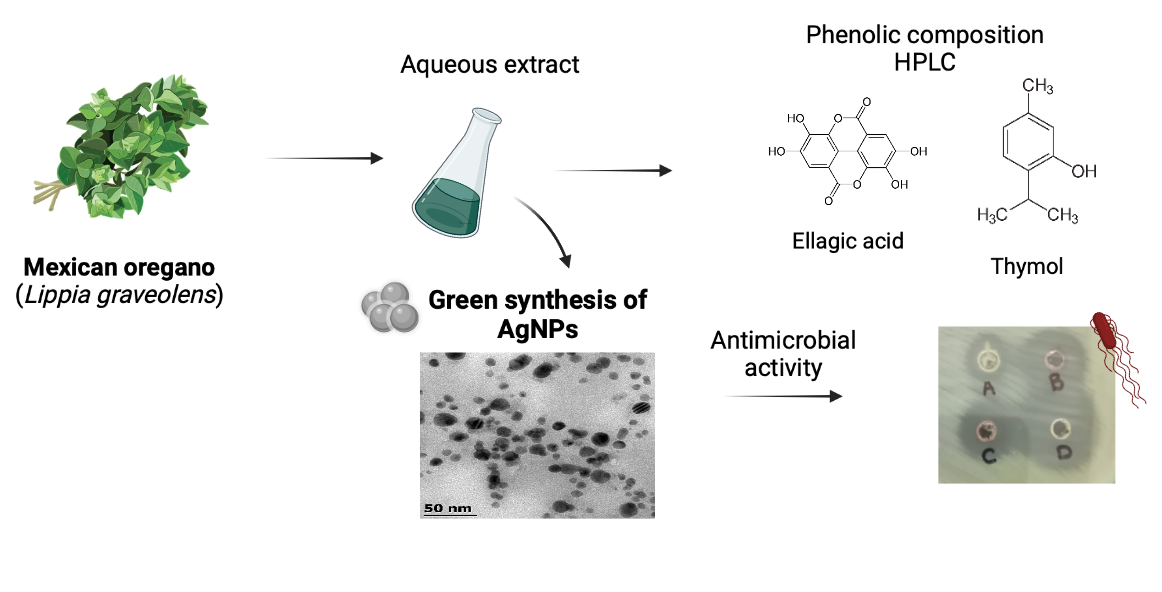
Abstract. The synthesis of silver nanoparticles (AgNPs) with plant extracts has acquired a lot of interest in recent years, due to its different applications in areas such as medicine, optics, food, pharmaceutic, among others. The aim of this work was to evaluate aqueous extracts of Mexican oregano (Lippia graveolens), rich in antioxidant compounds, to synthesize AgNPs. L. graveolens extract was characterized by HPLC and the antioxidant capacity was evaluated by ABTS, DPPH and CUPRAC. The effect of factors such as pH, concentration of precursor and temperature on the synthesis of AgNPs was studied. The particles were characterized by SEM, TEM, FTIR and their stability was evaluated with respect to time. The AgNps showed a spherical shape with an average diameter of 2.4 nm, and antimicrobial activity against S. aureus, L. monocytogenes and E. coli. After 30 days of storage, the AgNps agglomerated to form dendritic structures.
Resumen. La síntesis de nanopartículas de plata (AgNPs) mediante extractos de plantas ha adquirido interés en años recientes debido a los diversos campos donde pueden usarse, como la medicina, óptica, alimentos, farmacéutica, entre otras. El objetivo de esta investigación fue evaluar la capacidad de extractos acuosos del orégano mexicano (Lippia graveolens), rico en compuestos antioxidantes, para sintetizar AgNPs. El extracto de L. graveolens fue caracterizado por HPLC y la actividad antioxidante fue evaluada mediante los ensayos de ABTS, DPPH y CUPRAC. Se estudió el efecto del pH, concentraciones de precursor, y temperatura en la síntesis de AgNPs. Las partículas fueron caracterizadas mediante SEM, TEM, FTIR y su estabilidad con respecto al tiempo fue evaluada. Las AgNps presentaron una forma esférica con diámetro promedio de 2.4 nm, y actividad antimicrobiana contra S. aureus, L. monocytogenes and E. coli. Después de 30 días de almacenaje, las AgNps se aglomeraron formando estructuras dendriticas.
Downloads
References
Ahmad, S.; Munir, S.; Zeb, N.; Ullah, A.; Khan, B.; Ali, J.; Bilal, M.; Omer, M.; Alamzeb, M.; Salman, S. M.; Ali, S. Int. J. Nanomedicine. 2019, 14, 5087–5107. DOI: https://doi.org/10.2147/IJN.S200254.
Saha, J.; Begum, A.; Mukherjee, A.; Kumar, S. Sustain. Environ. Res. 2017, 27, 245–250. DOI: https://doi.org/10.1016/j.serj.2017.04.003.
Sharma, V.; Kaushik, S.; Pandit, P.; Dhull, D.; Yadav, J. P.; Kaushik, S. Appl. Microbiol. Biotechnol. 2019, 103, 881–891. DOI: https://doi.org/10.1007/s00253-018-9488-1.
Pattanayak, S.; Rahaman, M.; Maity, D.; Chakraborty, S.; Kumar, S.; Chattopadhyay, S. J. Saudi Chem. Soc. 2017, 21, 673–684. DOI: https://doi.org/10.1016/j.jscs.2015.11.004.
Sudha, A.; Jeyakanthan, J.; Srinivasan, P. Resource-Efficient Technologies. 2017, 3, 506-515. DOI: https://doi.org/10.1016/j.reffit.2017.07.002.
Castillo-Henríquez, L.; Alfaro-Aguilar, K.; Ugalde-álvarez, J.; Vega-Fernández, L.; de Oca-Vásquez, G. M.; Vega-Baudrit, J. R. Nanomater. 2020, 10, 1–24. DOI: https://doi.org/10.3390/nano10091763
Kumar, A.; Chisti, Y.; Chand, U. Biotechnol. Adv. 2013, 31, 346–356. DOI: https://doi.org/10.1016/j.biotechadv.2013.01.003.
Alshameri, A.W.; Owais, M. OpenNano. 2022, 8, 100077. DOI: https://doi.org/10.1016/j.onano.2022.100077.
Hasan, K. M.; Xiaoyi, L.; Shaoqin, Z.; Horváth, P. G.; Bak, M.; Bejó, L.; Sipos, G.; Alpár, T. Heliyon. 2022, 8, e12322. DOI: https://doi.org/10.1016/j.heliyon.2022.e12322.
Nadaf, S.J.; Jadhav, N. R.; Naikwadi, H. S.; Savekar, P. L.; Sapkal, I.D.; Kambli, M.M.; Desai, I. A. OpenNano. 2022, 8, 100076. DOI: https://doi.org/10.1016/j.onano.2022.100076.
Gopinath, K.; Venkatesh, K. S.; Ilangovan, R.; Sankaranarayanan, K.; Arumugam, A. Ind. Crops Produc. 2013, 50, 737–742. DOI: https://doi.org/10.1016/j.indcrop.2013.08.060.
Sulaiman, G. M.; Mohammed, W. H.; Marzoog, T. R.; Al-, A. A. A.; Kadhum, A. A. H. ; Mohamad, A. B. Asian Pac. J. Trop. Biomed. 2013, 3, 58–63. DOI: https://doi.org/10.1016/S2221-1691(13)60024-6.
Sankar, R., Karthik, A., Prabu, A., Karthik, S., Subramanian, K., Ravikumar, V. Colloids Surf. B: Biointerfaces, 2013, 108, 80–84. DOI: https://doi.org/10.1016/j.colsurfb.2013.02.033.
Hambardzumyan, S.; Sahakyan, N.; Petrosyan, M.; Nasim, M. J. ; Jacob, C. ; Trchounian, A. AMB Express. 2020, 10. DOI: https://doi.org/10.1186/s13568-020-01100-9.
Martínez-Rocha, A.; Puga, R.; Hernández-Sandoval, L.; Loarca-Piña, G.; Mendoza, S. Plant Foods Hum. Nutr. 2008, 63, 1–5 DOI: https://doi.org/10.1007/s11130-007-0061-9.
González-Fuentes, F.; López-Gil, M.A.; Mendoza S.; Escarpa A. Electroanalysis. 2011, 9, 2212–2216. DOI: https://doi.org/10.1002/elan.201100245.
Cortés-Chitala, M. D. C.; Flores-Martínez, H.; Orozco-Ávila, I.; León-Campos, C.; Suárez-Jacobo, Á.; Estarrón-Espinosa, M.; López-Muraira, I. Molecules. 2021, 26. DOI: https://doi.org/10.3390/molecules26030702.
Soto-Domínguez, A.; García-Garza, R.; Ramírez-Casas, Y.; Morán-Martínez, J.; Serrano-Gallardo, L. B. Int. J. Morphol. 2012, 30, 937–944. DOI: https://doi.org/10.4067/S0717-95022012000300029.
Cárdenas, A.; Gómez, M.; Frontana, C. Electrochim. Acta. 2014, 128, 113–118. DOI: https://doi.org/10.1016/j.electacta.2013.10.191.
Fukumoto, L. R.; Mazza, G. J. Agric. Food Chem. 2000, 48, 3597–3604. DOI: https://doi.org/10.1021/jf000220w.
Nenadis, N.; Wang, L. F.; Tsimidou, M.; Zhang, H. Y. J. Agric. Food Chem. 2004, 52, 4669–4674. DOI: https://doi.org/10.1021/jf0400056.
Rodríguez, B. A.; Mendoza, S.; Iturriga, M. H.; Castaño-Tostado, E. J. Food Sci. 2012, 77, C121-C1217. DOI: https://doi.org/10.1111/j.1750-3841.2011.02487.x.
Ramírez-Jiménez, A. K.; Reynoso-Camacho, R.; Mendoza-Díaz, S.; Loarca-Piña, G. Food Chem. 2014, 161, 254–260. DOI: https://doi.org/10.1016/j.foodchem.2014.04.008.
Soto, K.M.; Quezada-Cervantes, C.T.; Hernández-Iturriaga, M.; Luna-Bárcenas, G.; Vazquez-Duhalt, R.; Mendoza, S. LWT - Food Sci. Technol. 2019, 103, 293–300. DOI: https://doi.org/10.1016/j.lwt.2019.01.023.
Herrera-Rodríguez, S. E.; López-Rivera, R. J.; García-Márquez, E.; Estarrón-Espinosa, M.; Espinosa-Andrews, H. Food Sci. Biotechnol. 2019, 28, 441–448. DOI: https://doi.org/10.1007/s10068-018-0499-6.
Skendi, A.; Irakli, M.; Chatzopoulou, P. J. Appl. Res. Med. Aromat. Plants. 2017, 6, 62–69. DOI: https://doi.org/10.1016/j.jarmap.2017.02.001.
Bendini, A.; Dell, V.; Antiossidante, A.; Foglie, D. I.; Origano, D. I. Biol. Nyssana. 2016, 7, 131–139. DOI: https://doi.org/10.5281/zenodo.200410.
Boroski, M.; Giroux, H.J.; Sabik, H.; Petit, H.V.; Visentainer, J.V.; Matumoto-Pintro, P.T.; Britten, M. LWT - Food Sci. Technol. 2012, 47, 167-174. DOI: https://doi.org/10.1016/j.lwt.2011.12.018.
Cortés-Chilata, M. C.; Flores-Martínez, H.; Orozco-Ávila, I.; León-Campos, C.; Suárez-Jacobo, A.; Estarrón-Espinosa, M.; López-Muraira, I.; Molecules 2021, 26, 702. DOI: https://doi.org/10.3390/molecules26030702.
Heydari, R.; Rashidipour, M. Int. J. Breast Cancer. 2015, 1. DOI: https://doi.org/10.1155/2015/846743.
Hamouda, R. A.; Hussein, M. H.; Abo-Elmagd, R. A.; Bawazir, S. S. Sci. Rep. 2019, 9, 1–17. DOI: https://doi.org/10.1038/s41598-019-49444-y.
Kumar, M.; Bansal, K.; Gondil, V. S.; Sharma, S.; Jain, D. V. S.; Chhibber, S.; Sharma, R. K.; Wangoo, N. J. Mol. Liq. 2018, 249, 1145–1150. DOI: https://doi.org/10.1016/j.molliq.2017.11.143.
Liu, H.; Zhang, H.; Wang, J.; Wei, J. Arab. J. Chem. 2017, 13,1011-1019. DOI: https://doi.org/10.1016/j.arabjc.2017.09.004.
Verma, A.; Mehata, M. S. J. Radiat. Res. Appl. Sci. 2016, 9, 109–115. DOI: https://doi.org/10.1016/j.jrras.2015.11.001.
Ashkarran, A.; Bayat, A. Int. Nano Lett. 2013, 3, 50. DOI: https://doi.org/10.1186/2228-5326-3-50.
Huang, X.; El-Sayed, M. A. J. Adv. Res. 2010, 1, 13–28 DOI: https://doi.org/10.1016/j.jare.2010.02.002.
Marciniak, L.; Nowak, M.; Trojanowska, A.; Tylkowski, B.; Jastrzab, R. Materials. 2020, 13, 1–12. DOI: https://doi.org/10.3390/ma13235444.
Rawat, V.; Sharma, A.; Bhatt, V.P.; Singh, R.P.; Maurya, I.K. Mater. Today Proc. 2020, 29, 911–916. DOI: https://doi.org/10.1016/j.matpr.2020.05.274.
Khan, M.; Tareq, F.; Hossen, M.A.; Roki, M.N.A.M. J. Eng. Sci. Technol. 2018, 13, 158–166. DOI: https://www.researchgate.net/publication/322731405.
Pallela, P.N.V.K.; Ummey, S.; Ruddaraju, L.K.; Pammi, S.V.N.; Yoon, S.G. Microb. Pathog. 2018, 124, 63–69. DOI: https://doi.org/10.1016/j.micpath.2018.08.026.
Amaliyah, S.; Sabarudin, A.; Masruri, M.; Sumitro, S.B. Appl. Pharm. Sci. 2022, 12, 103–114. DOI: https://doi.org/10.7324/JAPS.2022.120311.
Otunola, G.; Afolayan, A.; Ajayi, E.; Odeyemi, S. Pharmacogn. Mag. 2017, 13, 201. DOI: 10.4103/pm.pm_430_16. DOI: https://doi.org/10.4103/pm.pm_430_16
Black, C.; Haughey, S. A.; Chevallier, O. P.; Galvin-King, P.; Elliott, C. T. Food Chem. 2016, 210, 551–557. DOI: https://doi.org/10.1016/j.foodchem.2016.05.004.
Barbieri, N.; Sanchez-Contreras, A.; Canto, A.; Cauich-Rodriguez, J. V.; Vargas-Coronado, R.; Calvo-Irabien, L. M. Ind. Crops Prod. 2018, 121, 114–123. DOI: https://doi.org/10.1016/j.indcrop.2018.04.081.
Li, R.; Chen, Z.; Ren, N.; Wang, Y.; Wang, Y.; Yu, F. J. Photochem.Photobiol. B: Biol. 2019, 199, 111593. DOI: https://doi.org/10.1016/j.jphotobiol.2019.111593.
Zhang, X.; Gao, C.; Lü, S.; Duan, H.; Jing, N.; Dong, D.; Shi, C.; Liu, M. J. Mater. Chem. B, 2014, 2, 5452–5460. DOI: https://doi.org/10.1039/c4tb00905c.
Nersisyan, H. H.; Lee, Y. J.; Joo, S. H.; Han, S. K.; Lee, T. H.; Lee, J. S.; An, Y. S.; Lee, J. H. Cryst. Eng. Comm. 2015, 17, 7535–7542. DOI: https://doi.org/10.1039/c5ce01367d.
Carbone, K.; Paliotta, M.; Micheli, L.; Mazzuca, C.; Cacciotti, I., Nocente, F.; Ciampa, A.; Dell’Abate, M. T. Arab. J. Chem. 2019, 12, 597–609. DOI: https://doi.org/10.1016/j.arabjc.2018.08.001.
Vimbela, G. V.; Ngo, S. M.; Fraze, C.; Yang, L.; Stout, D. A. Int. J. Nanomed. 2017, 12, 3941–3965. DOI: https://doi.org/10.2147/IJN.S134526.
Ruíz-Baltazar, Á. D. J.; Reyes-lópez, S. Y.; Larrañaga, D.; Estévez, M.; Pérez. R. Results Phys. 2017, 7, 2639–2643. DOI: https://doi.org/10.1016/j.rinp.2017.07.044.
Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. J. Nanomater. 2015, 1. DOI: https://doi.org/10.1155/2015/720654.
Arana-Sánchez, A.; Estarrón-Espinosa, M.; Obledo-Vázquez, E. N.; Padilla-Camberos, E.; Silva-Vázquez, R.; Lugo-Cervantes, E. Lett. Appl Microbiol. 2010, 50, 585–590. DOI: https://doi.org/10.1111/j.1472-765X.2010.02837.x.
Rueda, E.; Juvera, J.; Romo, I.; Holguín, R. Rev. Mexicana Cienc. Agri. 2018, 20, 4251–4261. DOI: http://www.scielo.org.mx/pdf/remexca/v9nspe20/2007-0934-remexca-9-spe20-4251-en.pdf.

Downloads
Published
Issue
Section
License
Copyright (c) 2024 Karen M. Soto, Montserrat Hernández-Iturriaga, Arely Cárdenas, Sandra Mendoza

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









