4(S)-Benzyl-1,3-thiazolidin-2-one a Novel Chiral Auxiliary for Asymmetric Aldol Coupling through Titanium Enolates
DOI:
https://doi.org/10.29356/jmcs.v68i1.2067Keywords:
Chiral auxiliaries, Coupling reaction, Stereoselective synthesis, Aldol adducts, ThiazolidinoneAbstract
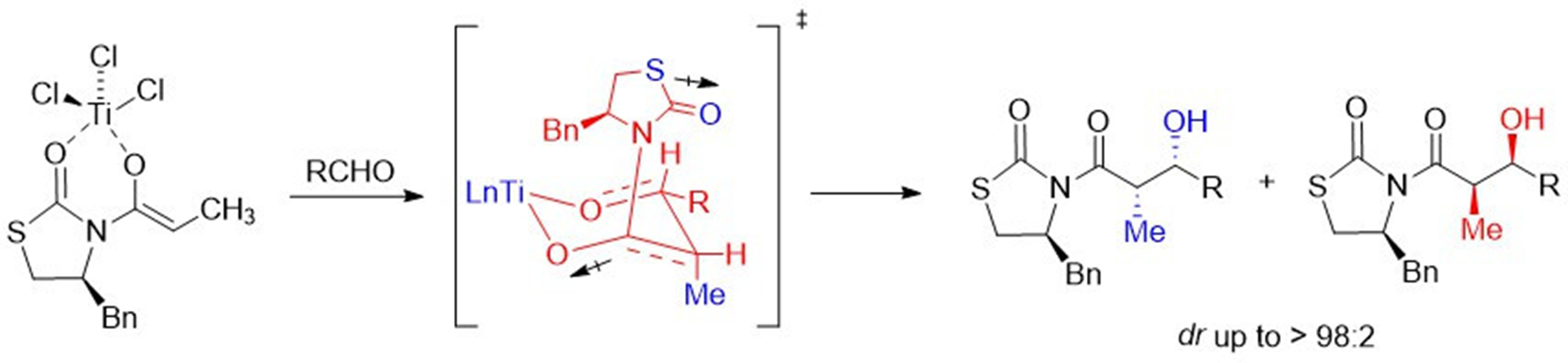
The chlorotitanium enolate of N-propionyl-4(S)-benzyl-1,3-thiazolidin-2-one, was condensed with aryl aldehydes, in good diastereoselectivity to give the ‘Evans syn’ aldol (73:27 - 97:3), using equimolar amounts of titanium tetrachloride (1.5 equiv), and N,N-diisopropylethylamine (DIPEA). The facial selectivity in the aldol additions probably involves a non-chelated transition state. In all aldol reactions, the presence of a minor product, the ‘non-Evans syn’ aldol, was obtained and confirmed by X-ray diffraction analysis of a single-crystal compound containing the mixture of diastereoisomers. The chiral auxiliary in these 1,3-thiazolidin-2-one aldol derivatives can be easily removed by nucleophilic species through acyl substitution.
Resumen. El enolato de clorotitanio de N-propionil-4(S)-bencil-1,3-tiazolidin-2-ona, fue condensado con arilaldehídos, con buena diastereoselectividad produciendo el aldol “syn Evans” (73:27 - 97:3), utilizando cantidades equimolares de tetracloruro de titanio (1.5 equiv) y N,N-diisopropiletilamina (DIPEA). La selectividad facial en las adiciones aldólicas probablemente implica un estado de transición no-quelatado. En todas las reacciones aldólicas, la presencia de un producto minoritario, él aldol “non-Evans”, fue obtenido y confirmado por el análisis de difracción de rayos-X de monocristal de una mezcla de los diastereoisómeros. El auxiliar quiral en estos derivados de 1,3-tiazolidin-2-onas puede ser removido fácilmente por especies nucleofílicas a través de la sustitución del grupo acilo.
Downloads
References
(a) Anger D.J.: Prakash, I.: Schaad, D.R. Aldrichim. Acta 1997, 30, 3-12. (a) Anger D. J.: Prakash, I.: Schaad, D. R. Aldrichim. Acta 1997, 30, 3-12, (b) Evans, D. A.; Bartoli, J.; Shih, T. L. J. Am. Chem. Soc. 1981, 103, 2127–2129. (c) Evans, D. A.; Ennis, M. D.; Mathre, D. J. J. Am. Chem. Soc. 1982, 104, 1737–1739. (d) Evans, D. A.; Chapman, K. T.; Bisaha, J. J. Am. Chem. Soc. 1984, 106, 4261–4263. (e) Evans D.A.; J.S. Clark, R. Mettemich, V.J. Novack, G.S. Sheppard, J. Am. Chem. Soc. 1990, 112, 866.
(a) Oppolzer, W.; Blagg, J.; Rodriguez, I.; Walther, E. J. Am. Chem. Soc. 1990, 112, 2767-2772. (b) Ortiz, A.; Sansinenea, E. J. Sulf. Chem. 2007, 28, 109-147. (c) Crimmins, M. T. King, B.W.; Tabet, E. A. J. Am. Chem. Soc. 1997, 119, 7883-7884. (d) Ortiz, A.; Sansinenea, E. J. Sulf. Chem. 2007, 28, 109-147.
(a) Velázquez, F.; Olivo, H.F. Curr. Org. Chem. 2002, 6, 1-38, (b) Yan, T.-H.; Tan, C.-W.; Lee, H.-C.; Lo, H.-C.; Huang, T.-Y. J. Am. Chem. Soc. 1993, 115, 2613-2621, (c) T.-H.; Hung, A.-W.; Lee, H.-C.; Chang, C.-S.; Liu, W.-H. J. Org. Chem. 1995, 60, 3301-3306. (d) Crimmins, M. T.; King, B. W.; Tabet, E. A. J. Am. Chem. Soc., 1997, 119, 7883-7884.
Farina, V.; Reeves, J.T.; Senanayake, C.H.; Song, J. J. Chem. Rev. 2006, 106, 2734-2793. DOI: https://doi.org/10.1021/cr040700c
Crimmins, M.T.; King, B.W.; Tabet, E.A.; Chaudhary, K. J. Org. Chem. 2001, 66, 894-902.
Evans, D.A.; Bartroli, J.; Shih, T.L. J. Am. Chem. Soc. 1981, 103, 2127–2129.
Evans D.A.; Nelson, J.V.; Vogel, E.; Taber, T.R. J. Am. Chem. Soc. 1981, 103, 3099–3111. DOI: https://doi.org/10.1021/ja00401a031
Evans, D.A.; Rieger, D.L.; Bilodeau, M.T.; Urpi, F. J. Am. Chem. Soc. 1991, 113, 1047–1049. DOI: https://doi.org/10.1021/ja00003a051
Walker, M.A.; Heathcock, C. H. J. Org. Chem. 1991, 56, 5747–575. DOI: https://doi.org/10.1021/jo00020a006
Crimmins M.T.; King, B. W.; Tabet, E. A.; Chaudhary, K. J. Org. Chem. 2001, 66, 894–902. DOI: https://doi.org/10.1021/jo001387r
Nagao, Y.; Yamada, S.; Kumagai, T.; Ochiai, M.; Fujita, E. J. Chem. Soc. Chem. Commun. 1985, 1418–1419. DOI: https://doi.org/10.1039/C39850001419
Alexakis, A.; Bäckwall, J.E.; Krause, N.; Pamies, O.; Dieguez, M. Chem. Rev. 2008, 108, 2796-2823. DOI: https://doi.org/10.1021/cr0683515
(a) Williams, D.R.; Nold, A.L.; Mullins, R.J. J. Org. Chem. 2004, 69, 5374-5382. (b) Esumi, T.; Shimizu, H.; Kashiyama, A.; Sasak C.; Toyota, M. Tetrahedron Lett. 2008, 49, 6846-6849.
(a) Kitajima, H.; Ito, K.; Katsuki, T. Tetrahedron. 1997, 53, 17015–17028; (b) Krause, N.; Hoffmann-Roeder, A. Synthesis. 2001, 171–196
(a) Crimmins, M. T.; Chaudhary, K. Org. Lett. 2000, 2, 775–777; (b) Crimmins, M. T.; Caussanel, F. J. Am. Chem. Soc. 2006, 128, 3128–3129; (c) Crimmins, M. T.; Slade, D. J. Org. Lett. 2006, 8, 2192–2194.
(a) Palomo, C.; Oiarbide, M.; Dias, F.; Ortiz, A.; Linden, A. J. Am. Chem. Soc. 2001, 123, 5602. (b) Ortiz, A.; Quintero, L.; Hernandez, H.; Maldonado, S.; Mendoza, G.; Bernes, S. Tetrahedron Lett. 2003, 44, 1129. (c) Palomo, C.; Oiarbide, M.; Lopez, R.; Gonzalez, P. B.; Gomez-Bengoa, E.; Sa, J. M.; Linden, A. J. Am. Chem. Soc. 2006, 128, 15236.
Amagata, T.; Johnson, T. A.; Cichewicz, R. H.; Tenney, K.; Mooberry, S. L.; Media, J.; Edelstein, M.; Valeriote, F. A.; Crews, P. J. Med. Chem. 2008, 51, 7234–7242. DOI: https://doi.org/10.1021/jm8008585
Sonnenschein, R. N.; Johnson, T. A.; Tenney, K.; Valeriote, F. A.; Crews, P. J. Nat. Prod. 2006, 69, 145–147. DOI: https://doi.org/10.1021/np0503597
(a) Shibahara, F.; Suenami, A.; Yoshida, A.; Murai, T. Chem. Commun. 2007, 2354. (b) Mohammadpoor-Baltork, I.; Khodaei, M. M.; Nikoofar, K. Tetrahedron Lett. 2003, 44, 591. (c) Kim, Y. H.; Chung, B. C.; Chang, S. Tetrahedron Lett. 1985, 26, 1079. (d) Jorgensen, K. A.; El-Wassimy, M. T. M.; Lawesson, S. O. Tetrahedron. 1983, 39, 469. (e) Kochhar, K. S.; Cottrell, D. A.; Pinnick, H. W. Tetrahedron Lett. 1983, 24, 1323.
(a) Liu, Z. G.; Dai, C. S. Acta Chim. Sinica 1965, 31, 258–259; (b) Zheng, B.; Yang, Q. C.; Li, L. Chinese patent, CN 1403447, 2003.
(a) Moreno-Manas, M.; Padros, I. J. Heterocycl. Chem. 1993, 30, 1235–1239; (b) Kitoha, S.; Kubotab, A.; Kunimotoa, K.; Kuwaec, A.; Hanaic, K. J. Mol. Struct. 2005, 737, 277–282
Deng, X.; Chen, N.; Wang, Z.; Li, X.; Hu, H.; Xu, J. Phosphorus, Sulfur Silicon 2011, 186, 1563. DOI: https://doi.org/10.1080/10426507.2010.525770
Romero, O.; Minor-Villar. L.; Olivo, H.; Synlett, 2012, 23, 2835 2839. DOI: https://doi.org/10.1055/s-0032-1317525
Evans, D. A.; Bartroli, J.; Shih, T. L. J. Am. Chem. Soc. 1981, 103, 2127-2129. DOI: https://doi.org/10.1021/ja00398a058
D. Delaunay, L. Toupet, M.L. Corre, J. Org. Chem. 1995, 60, 6604. DOI: https://doi.org/10.1021/jo00125a059
Crimmins, M. T. Chaudhary, K. Org. Lett. 2000, 2, 775-777. DOI: https://doi.org/10.1021/ol9913901
(a) Flack, H. D.; Bemardinelli, G. Acta Cryst. A 1999, 55, 908-915. (b) Flack H. D.; Bemardinelli G. J. Appl Cryst. 2000, 33, 1143-1148.
CCDC 1839074 (4a), 1839075 (6a), 1839076 (6b), 1839077 (6c), 1839078 (6d), 1839079 (6f) 1839080 (6g), 1839081 (6h). https://www.ccdc.cam.ac.uk/structures/?, Accessed December 14, 2023.
Evans, D. A. In Proceedings of The Robert A. Welch Foundation Conferences on Chemical Research. XXXVII. Stereospecificity in Chemistry and Biochemistry; Ed. Welch Foundation, 1984.

Downloads
Additional Files
Published
Issue
Section
License
Copyright (c) 2023 Dulce, Salvador Mastachi-Loza, Diego, Moises

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









