Synthesis and NMR characterization of Bile Acid Derivatives Bearing Ugi 4CR-Modified Side Chains
DOI:
https://doi.org/10.29356/jmcs.v68i2.2061Keywords:
Bile acids, Ugi 4CR, 1H NMR, 13C{1H} NMRAbstract
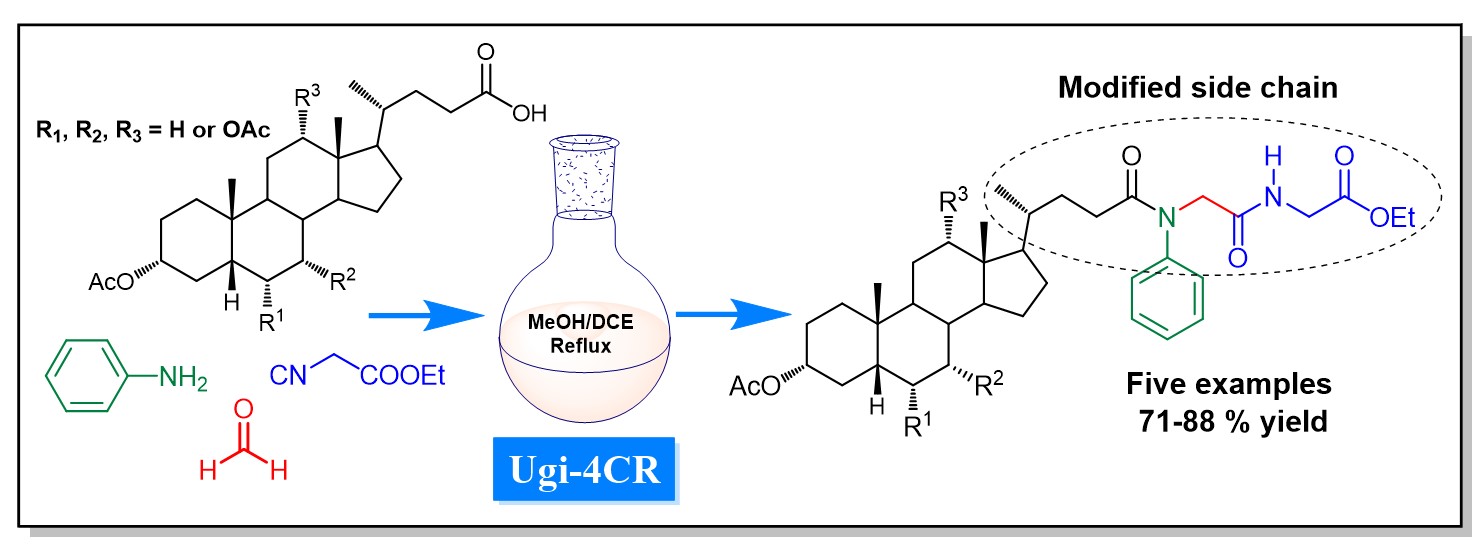
Abstract. The application of the four-component Ugi reaction for the synthesis of five bile acid derivatives bearing modified side chains is described. The unambiguous structural characterization and assignment of the functional 1H NMR signals and all 13C{1H} NMR chemical shifts are presented.
Resumen. Se describe la aplicación de la reacción de cuatro componentes de Ugi a la síntesis de cinco derivados de ácidos biliares que portan cadenas laterales modificadas. Se presenta la caracterización estructural inequívoca y la asignación de las señales funcionales de 1H RMN y de todos los desplazamientos químicos de 13C{1H} RMN.
Downloads
References
Monte, M. J.; Marin, J. J.; Antelo, A.; Vazquez-Tato, J. World J. Gastroenterol. 2009, 15, 804−816. DOI: dx.doi.org/10.3748/wjg.15.804. DOI: https://doi.org/10.3748/wjg.15.804
Virtanen, E.; Kolehmainen, E. Eur. J. Org. Chem. 2004, 3385−3399. DOI: doi.org/10.1002/ejoc.200300699. DOI: https://doi.org/10.1002/ejoc.200300699
(a) Luo, J.; Chem, Y.; Zhu, X.X. Langmuir. 2009, 25, 10913–10917. DOI: doi.org/10.1021/la9013703. (b) Maitra, U.; Mukhopadhyay, S.; Sarkar, A.; Rao, P.; Indi, S. S. Angew. Chem., Int. Ed. 2001, 40, 2281−2283. DOI: doi.org/10.1002/1521-3773(20010618)40:12<2281::AID-ANIE2281>3.0.CO;2-L . (c) Zhang, J.; Luo, J.; Zhu, X.X.; Junk, M. J. N.; Hinderberger, D. Langmuir. 2010, 26, 2958–2962. DOI: doi.org/10.1021/la9028996.
Brotherhood, P. R.; Davis, A. P. Chem. Soc. Rev., 2010, 39, 3633–3647. DOI: doi.org/10.1039/B926225N. DOI: https://doi.org/10.1039/b926225n
Zhang, M.; Strandman, S.; Waldron, K. C.; Zhu, X. X. J. Mater. Chem. B, 2016, 4, 7506–7520. DOI: doi.org/10.1039/C6TB02270G. DOI: https://doi.org/10.1039/C6TB02270G
(a) Li, R.; Carpentier, E., Newell, E.D.; Olague L.M.; Heafey, E.; Yihwa, C. Langmuir. 2009, 25, 13800–13808. DOI: doi.org/10.1021/la901826y. (b) Santiago-Sampedro, G. I.; Aguilar-Granda, A.; Torres-Huerta, A.; Flores-Álamo, M.; Maldonado-Domínguez, M.; Rodríguez-Molina, B.; Iglesias-Arteaga, M. A. J. Org. Chem. 2022, 87, 2255−2266. DOI: doi.org/10.1021/acs.joc.1c01334.
Mayorquín-Torres, M.C.; Flores-Alamo, M.; Iglesias-Arteaga, M.A. Steroids, 2015, 101, 21–27. DOI: https://doi.org/10.1016/j.steroids.2015.05.007
Selected references (a) Zhu, J.; Bienaymé, H. Multicomponent Reactions, 1st Ed. Wiley-VCH: Weinheim, Germany, 2005, 1−468. (b) Dömling, A.; Wang, W.; Wang, K. Chem. Rev. 2012, 112, 3083–3185. DOI: doi.org/10.1021/cr100233r. (c) Rotstein, B. H.; Zaretsky, S.; Rai, V.; Yudin, A. K. Chem. Rev. 2014, 114, 8323−8359. DOI: doi.org/10.1021/cr400615v. (d) Sunderhaus, J. D.; Martin, S. F. Chem. Eur. J. 2009, 15, 1300–1308. DOI: doi.org/10.1002/chem.200802140. (e) Biggs-Houck, J. E.; Younai, A.; Shaw, J. T. Curr. Op. Chem. Biol. 2010, 14, 371−382. DOI: doi.org/10.1016/j.cbpa.2010.03.003. (f) Ulaczyk-Lesanko, A.; Hall, D. G. Curr. Op. Chem. Biol. 2005, 9, 266−276. DOI: doi.org/10.1016/j.cbpa.2005.04.003. (g) Cioc, R. C.; Ruijter, E.; Orru, R. V. A. Green Chem. 2014, 16, 2958–295. DOI: https://doi.org/10.1039/C4GC00013G. (h) Sunderhaus, J. D.; Martin, S. F. Chem. Eur. J. 2009, 15, 1300–1308. DOI: https://doi.org/10.1002/chem.200802140. (i) Cores, A.; Clerigué, J.; Orocio-Rodríguez, M.; Menéndez, J. C. Pharmaceuticals. 2022, 15, 1009. DOI: https://doi.org/10.3390/ph15081009.
(a) Reguera, L.; Attorresi, C. I.; Ramírez, J. A.; Rivera, D. G. Beilstein J. Org. Chem. 2019, 15, 1236–1256. DOI: doi.org/10.3762/bjoc.15.121. (a) Rivera, D. G.; Wessjohann, L. A.; Molecules. 2007, 12, 1890-1899. DOI: doi.org/10.3390/12081890.
(a) Mayorquín-Torres, M. C.; Flores-Álamo, M.; Iglesias-Arteaga, M. A. Tetrahedron Lett. 2017, 58, 3500–3504. DOI: doi.org/10.1016/j.tetlet.2017.07.081. (b) Mayorquín-Torres, M. C.; Navarro-Huerta, A.; Maldonado-Domínguez, M.; Flores-Álamo, M.; Rodríguez-Molina, B.; Iglesias-Arteaga, M. A. J. Org. Chem. 2021, 86, 4112−4120. DOI: doi.org/10.1021/acs.joc.0c02943.
Selected references: (a) Rocha, O.R.; Rodrigues M.O.; Neto. B.A.D. ACS Omega. 2020, 5, 972–979. DOI: doi.org/10.1021/acsomega.9b03684. (b) Foucad, M. A.; Abdel-Hamid, H.; Ayoup M. S. RSC Adv. 2020, 10, 42644–42681. DOI: doi.org/10.1039/D0RA07501A.

Downloads
Additional Files
Published
Issue
Section
License
Copyright (c) 2024 Josué Vazquez-Chavez, Brian A. Verdeja-Perdomo, Martin A. Iglesias-Arteaga

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









