A Comparative Vibrational analysis, Electronic Properties, and molecular docking of Lantadene A and B (Potential anticancer agents) - A Computational DFT Study
DOI:
https://doi.org/10.29356/jmcs.v68i3.2060Keywords:
Lantadene A & B, Vibrational analysis, DFT, HOMO-LUMO & MESP, Molecular DockingAbstract
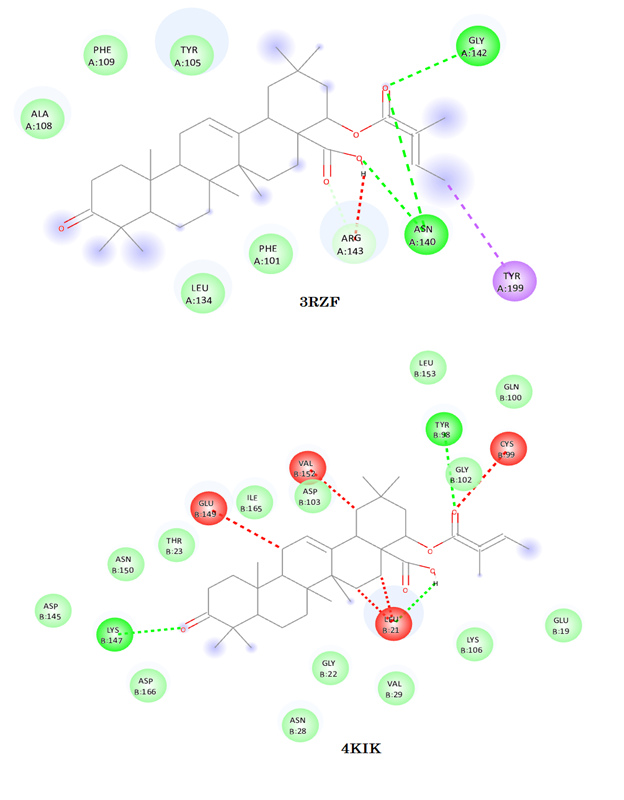
Abstract. We conducted a comprehensive analysis of Lantadene A and B using FTIR spectroscopy, beginning with geometry optimization. Subsequently, we calculated their fundamental vibrational frequencies and intensities using the B3LYP/6-311G (d, p) method. To provide a thorough vibrational assignment, we utilized potential energy distribution (PED). The results from our calculated spectra closely matched the experimental data, demonstrating the accuracy of our calculations. Furthermore, we assessed the electronic properties of Lantadene A and B. We computed the HOMO-LUMO gap and visualized the frontier orbital HOMO-LUMO surfaces, as well as Molecular Electrostatic Potential (MEP) surfaces. These analyses shed light on the reactive nature of these compounds, highlighting their potential applications. Moreover, our investigation explored the hyper-polarizability values, suggesting that Lantadene A and B hold promise for electro-optical applications due to their unique properties. Additionally, we conducted docking studies of Lantadene A and Lantadene B with BCL2L1 (BCL2 like 1) and IKBKB (inhibitor of nuclear factor kappa B kinase subunit beta) proteins, as provided by HGNC. These analyses revealed promising interactions, supporting the potential use of Lantadene A and B as agents with anti-cancer and anti-inflammatory properties. In summary, our research indicates that Lantadene A and B possess properties that make them strong candidates for use in the development of anticancer and anti-inflammatory agents, while also showing promise for electro-optical applications.
Resumen. Utilizando la espectroscopía de FTIR realizamos un análisis integral de lantadeno A y B, empezando con la optimización de sus geometrías. Después, calculamos las frecuencias e intensidades de vibración utilizando el método B3LYP/6-311G (d, p). Para realizar una asignación vibracional exhaustiva, utilizamos la distribución de energía potencial (PED). Los espectros calculados están en buen acuerdo con los experimentales, lo cual demuestra la precisión de nuestros cálculos. Además, evaluamos las propiedades electrónicas de lantadeno A y B. Calculamos la brecha (gap) HOMO-LUMO, visualizamos las isosuperficies de los orbitales frontera, y también las isosuperficies del potencial electrostático molecular (MEP). Estos análisis ayudan a esclarecer la reactividad de estas moléculas, destacando sus aplicaciones potenciales. Se exploraron los valores de las hiperpolizabilidades las cuales sugieren que el lantadeno A y B son compuestos prometedores para aplicaciones electroópticas. Adicionalmente, se realizaron estudios de acoplamiento molecular de lantadeno A y B con las proteínas BCL2L1 (BCL2 como 1) y IKBKB (inhibidor del factor kappa B de la subunidad beta quinasa), que se obtuvieron del HGNC. Estos análisis mostraron interacciones prometedoras, que apoyan el uso potencial de lantadeno A y B como agentes anticancerígenos y con propiedades antinflamatorias. En síntesis, nuestra investigación indica que las propiedades del lantadeno A y lantadeno B las hacen buenos candidatos para su uso en el desarrollo de agentes anticancerígenos y antinflamatorios, además de también mostrar potencial en aplicaciones electroópticas.
Downloads
References
Nadkami, A.K., Nadkami, K.M., Indian Materia Medica, Bombay Popular Prakashan, Bombay, 1976.
Guthikonda, R.N., Cama, L.D., Quesada, M., Woods, M.F., Salzmann, T.N., Christensen, B.G., Pure & Appl. Chem. 1987, 3, 455-458. DOI: http://dx.doi.org/10.1351/pac198759030455. DOI: https://doi.org/10.1351/pac198759030455
Ross, I. A., Medicinal plants of the world, Human Press, New Jersey, 1999. DOI: https://doi.org/10.1007/978-1-59259-365-1.
Kurian, A., K, Shankar M., Medicinal Plants-Horticulture Sciences, New India Publication Agency, India, 2007. DOI: https://doi.org/10.59317/9789389130959
The Wealth of India. The Council of Scientific and Industrial Research, Ind Med Gaz. 1949, 84(10), 476–477. PMCID: PMC5189551.
Vasantha, P., Sukumar, N., Sharma, O. P., Acta Cryst. 1991, C47, 810-812. DOI: https://doi.org/10.1107/S0108270190008897
Ghisalberti, E.L., Fitoterapia, 2000, 71, 467-485.DOI: http://doi.org/10.1016/s0367-326x(00)00202-1 DOI: https://doi.org/10.1016/S0367-326X(00)00202-1
Duke, J. A., Boca Raton, CRC Press, 1992.
Shashi, B.M., Niranjan, P.S., Subodh, K.R., Sharma, O.P., Tetrahedron, 1994, 50, 9439-9446. DOI: http://doi.org/10.1016/S0040-4020(01)85518-6. DOI: https://doi.org/10.1016/S0040-4020(01)85518-6
O' Neill, M.J., Lewis, J.A., Noble, H.M., Holland, S., Mansat, C., Farthing, J.E., Foster, G., Noble, D., Lane, S.J., Sidebottom, P.J., Lynn, S.M., Hayes, M.V., Dix, C.J., J. Nat. Prod., 1998, 61, 1328- 1331. DOI: http://doi.org/10.1021/np970464j. DOI: https://doi.org/10.1021/np970464j
Uzcategui, B., Avila, D., Heberto, S.R., Quintero, L., Ortega, J., Gonzalez, Y.B., Investigacion Clinica, 2004, 45, 4.
Misra, N., Sharma, M., Raj K., Dangi, A., Srivastava, S., Misra, S., Parasitol. Res., 2007, 100, 439-448. DOI: http://doi.org/10.1007/s00436-006-0312-y. DOI: https://doi.org/10.1007/s00436-006-0312-y
Begum, S., Wahab, A., Siddiqui, B., Qamar, F., Chem. Pharm. Bull. 2003, 51, 134–137. DOI: http://doi.org/10.1248/cpb.51.134. DOI: https://doi.org/10.1248/cpb.51.134
Zandi-Sohani, N., Hojjati, M., Carbonell, B., Angel, A., Chil. J. Agric. Res., 2012, 72, 502-506. DOI: http://dx.doi.org/10.4067/S0718-58392012000400007 DOI: https://doi.org/10.4067/S0718-58392012000400007
Shamsee, Z. R., Al-Saffar, A.Z., Al-Shanon, A.F., Al-Obaidi, J.R., Mol. Biol. Rep., 2019, 46, 381-390. DOI: http://doi.org/10.1007/s11033-018-4482-3. DOI: https://doi.org/10.1007/s11033-018-4482-3
Inada, Nakanishi, T., Tokuda, H., Nishino, H., Iwashina, A., and Sharma, O. P., Planta. Med., 1995, 61, 558. DOI: https://doi.org/10.1055/s-2006-959371
Inada, Nakanishi, T., Tokuda, H., Nishino, H., Iwashina, A., and Sharma, O. P., Planta. Med., 1997, 63, 272. DOI: https://doi.org/10.1055/s-2006-957673
Nethaji, M., Rufes, C., Sadasivao, C., Pattashi, V. Sharma, O., J. Crystallogr. Spectrosc. Res., 1993, 6, 469 472.
Goswami, G. A., Sawant, N., Biosci. Biotechnol. Res. Asia, 2011, 2, 821-824. https://www.biotech-asia.org/?p=9684.
Sharma, M., Sharma, P., Bansal, M., Indian J. Pharmacol., 2007, 39,140-144. DOI: https://doi.org/10.4103/0253-7613.33433
Sharma, M., Dalal, R. Sharma, N, Design Nat. Prod. Res., 2011, 4, 387-396. DOI: http://doi.org?10.1080/14786411003792173. DOI: https://doi.org/10.1080/14786411003792173
Dwivedi, A., Srivastava, A. K., Bajpai, A., Spectrochim. Acta, Part A., 2015, 149, 343-351. DOI: https://doi.org/10.1016/j.saa.2015.04.042.
Dwivedi, A., Pandey, A. K., Raj, K., Misra, N., Spectrosc. Int. J., 2012, 3, 155-166. DOI: http://doi.org/10.1155/2012/486304. DOI: https://doi.org/10.1155/2012/486304
Pandey, A. K., Siddiqui, S. A., Dwivedi, A., Raj, K., Misra, N., Spectrosc. Int. J., 2011, 25, 287-302. DOI: http://doi.org/10.3233/SPE-2011-0517. DOI: https://doi.org/10.1155/2011/361849
A. K. Pandey, A. Dwivedi, N. Misra, Spectrosc. Int. J., 2013. Article ID 937915, 11 pages. DOI: https://doi.org/10.1155/2013/937915.
Dwivedi, A., Kumar, A. Polycyclic Aromat. Compd., 2021, 41, 387-399. DOI: https://doi.org/10.1080/10406638.2019.1591466.
Becke, A.D., J. Chem. Phys, 1993, 98, 5648-5652. DOI: https://doi.org/10.1063/1.464913.
Lee, C., Yang, W., Parr, R.G. Phys. Rev. B., 1988, 37, 785. DOI: https://doi.org/10.1103/PhysRevB.37.785.
Frisch, M. J., et al Gaussian 09; Gaussian, Inc., Pittsburgh, PA, 2009.
Fast, P.L., Corchado, J., Sanches, M.L., Truhlar D.G., J. Phys.Chem. A. 1999, 103, 3139-3143. DOI: https://doi.org/10.1021/jp9900382.
Frisch, A., Nelson, A.B., Holder, A.J., Gauss view, Inc.Pittsburgh PA, 2000.
Jamroz M. H., Vibrational Energy Distribution Analysis: VEDA 4 program, Warsaw (2004).
Andersson, M.P., Uvdal, P. J. Phys. Chem. A, 2005, 12, 2937–2941. DOI: https://doi.org/10.1021/jp045733a.
Colthup, N.B., Daly, L.H., Wiberley, S.E., Introduction to Infrared and Raman Spectroscopy, Academic Press, New York, 1990.
Abraham, C.S., Muthu, S., Prasana, J.C., Armaković, S., Armaković, S.J., Geoffrey B., Spectrochim. Acta, Part A, 2019, 222, 117188. DOI: https://doi.org/10.1016/j.saa.2019.117188.
Thamarai, A., Vadamalar, R., Raja, M., Muthu, S., Narayana, B., Ramesh, P., R. Muhamed, R., Sevvanthi, S., Aayisha, S., Spectrochim. Acta, Part A, 2020, 226, 117609. DOI: https://doi.org/10.1016/j.saa.2019.117609.
Silverstein, R.M., Bassler, G.C., Morrill, T.C., Spectrometric Identification of Organic Compounds, 4th ed. John Wiley and Sons, New York, 1981.
Pulay, P., Fogarasi, G., Pang, F., Boggs, J.E., J. Am. Chem. Soc., 1979, 101, 2550–2560. DOI: https://doi.org/10.1021/ja00504a009.
Gutowski, M., Chalasinski, G., J. Chem. Phys. 1993, 98, 4540–4554.
Bose, S. C., Saleem, H., Erdogdu, Y., Rajarajan, G., Thanikachalam, V., Spectrochim. Acta, Part A, 2011, 82, 260–269. DOI: https://doi.org/10.1016/j.saa.2011.07.046.
Parr, R. G., Pearson, R.G., J. Am. Chem. Soc. 1983, 105, 7512–7516. DOI: https://doi.org/10.1021/ja00364a005.
Geerlings, P., Proft, F. D., Langenaeker, W., Chem. Rev. 2003, 103, 1793–1874. DOI: https://doi.org/10.1021/cr990029p
Parr, R.G., Donnelly, R.A., Levy, M., Palke, W.E., J. Chem. Phys., 1978, 68, 3801. DOI: https://doi.org/10.1063/1.436185.
Komorowski, L., Chem . Phys., 1987, 114, 55. DOI: https://doi.org/10.1016/0301-0104(87)80019-8
Parr, R.G., Szentpály, L., Liu, S., J. Am. Chem. Soc., 1999, 121, 1922–1924. DOI: https://doi.org/10.1021/ja983494x
Gadre, S.R., Pathak, R.K., J. Chem. Phys., 1990, 93, 1770–1774. DOI: https://doi.org/10.1063/1.459703.
Gadre, S.R., Shrivastava, I.H., J.Chem. Phys,. 1991, 94, 4384–4390. DOI: https://doi.org/10.1063/1.460625.
Murray J.S., Sen, K., Molecular Electrostatic Potentials, Concepts and Applications, Elsevier, Amsterdam, 1996.
Alkorta I., Perez, J.J., Int. J. Quant. Chem. 1996, 57, 123–135. DOI: https://doi.org/10.1002/(SICI)1097-461X(1996)57:1<123::AID-QUA14>3.0.CO;2-9
Matta, I.F., Boyd, R.J., The Quantum Theory of Atoms in Molecules, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2007.
Koch, U., Popelier, P., J. Phys. Chem. A, 1995, 99, 9747-9754. DOI: https://doi.org/10.1021/j100024a016.
Carroll, M. T., Bader, R. F. W., Mol. Phys., 1988, 65, 695. DOI: http://doi.org/10.1080/00268978800101351. DOI: https://doi.org/10.1080/00268978800101351
Cremer, D., Kraka, E., Croat.Chem. Acta., 1984, 57, 1259–1281. DOI: https://hrcak.srce.hr/194019
Rozas, I., Alkorta, I., Elguero, J., J. Am. Chem. Soc., 2000, 122, 11154–11161. DOI: https://doi.org/10.1021/ja0017864.
Bader, R. F. W., Atoms in Molecules: A Quantum Theory (2nd ed.) Oxford: New York, NY. 1990 DOI: https://doi.org/10.1093/oso/9780198551683.001.0001
Erdogdu, Y., Unsalan, O., Gulluoglu, M.T., J. Raman Spectrosc., 2010, 41, 820-828. DOI: https://doi.org/10.1002/jrs.2520
Erdogdu, Y., Unsalan, O., Amalanathan, M., Hubert, J. I., J. Mol. Struct., 2010, 980, 24-30. DOI: https://doi.org/10.1016/j.molstruc.2010.06.032
Gonohe, N., Abe, H., Mikami, N., Ito, M., J. Phys. Chem., 1985, 89, 3642-3648. DOI: https://doi.org/10.1016/0009-2614(83)85053-2 DOI: https://doi.org/10.1021/j100263a015
Alyar, H., Kantarci, Z., Bahat, M., Kasap, E., J. Mol. Struct., 2007, 834, 516-520. DOI: http://doi.org/10.1016/j.molstruc.2006.11.066. DOI: https://doi.org/10.1016/j.molstruc.2006.11.066
Padrón, J.A., Carasco, R., Pellón, R.F., J. Pharm. Pharmaceut. Sci., 2002, 5, 258–266.
Verma, R.P., Hansch, C., Bioorg. Med. Chem., 2005, 13 2355–2372. Doi: https://doi.org/10.1016/j.bmc.2007.01.011. DOI: https://doi.org/10.1016/j.bmc.2005.01.051
Verma, R.P., Kurup, A., Hansch, C., Bioorg. Med. Chem., 2005, 13, 237–255. DOI: https://doi.org/10.1016/j.bmc.2004.09.039
Vuks, M.F., Opt. Spectrosc., 1966, 20, 361-368. DOI: https://doi.org/10.1090/S0025-5718-1966-0210285-0
Kumar, A., Srivastava, A.K., Tiwari, S.N., Misra, N., Sharma, D., Mol. Cryst. Liq. Cryst., 2019, 1, 23–31. DOI: https://doi.org/10.1080/15421406.2019.1641987
Morris, G.M., Huey, R., Lindstrom, W., Sanner, M.F., Belew, R.K., Goodsell, D.S., Olson, A.J., J. Comput. Chem., 2009, 16, 2785–2791. DOI: https://doi.org/10.1002/jcc.21256
Pettersen, E.F., Goddard, T.D., Huang, C.C., Couch, G.S., Greenblatt, D.M., Meng, E.C., Ferrin, T.E., J. Comput. Chem. 2004, 25, 1605–1612. DOI: http://doi.org/10.1002/jcc.20084 DOI: https://doi.org/10.1002/jcc.20084
Morris, G.M., Goodsell, D.S., Halliday, R.S., Huey, R., Hart, W.E., Belew, R.K., Olson, A.J., J. Comput. Chem., 1998, 19, 1639–1662. DOI: https://doi.org/10.1002/(SICI)1096987X(19981115)19:14%3C1639::AID-JCC10%3E3.0.CO;2-B. DOI: https://doi.org/10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B
Discovery Studio 4.5 Guide, Accelrys Inc., San Diego, 2009. http://www. accelrys.com
Oost, M., Belli, T.K., Ding, B.A., Joseph, H., Kunzer, M.K., Martineau, A., McClellan, D., Mitten, W.J., et.al, J. Med. Chem., 2007, 50, 641-662. https://files.rcsb.org/view/2O2M.cif. DOI: https://doi.org/10.1021/jm061152t
Swain, S.S., Singh, S.R., Sahoo, A., Hussain, T., Pati, S., J. Biomol. Struc. Dyn., 2021, 40, 6463–6476. DOI: https://doi.org/10.1080/07391102.2021.1885495
www.swissdock.ch
Sillars-Hardebol, A. H., Carvalho, B., Beliën, J., Wit, M., Delis-van P. M. et. al., The Journal of pathology, 2012, 226, 3, 442-450. DOI: http://doi.org/10.1002/path.2983 DOI: https://doi.org/10.1002/path.2983
Kvansakul, M., Hinds, M.G., Banjara, S., Biochem. J., 2020, 477, 3287- 3297. DOI: https://doi.org/10.1042/BCJ20200556
https://files.rcsb.org/view/6WH0.cif.
https://files.rcsb.org/view/6WGZ.
Herrmann, O., Baumann, B., de Lorenzi, R., Muhammad, S., Zhang, W., Kleesiek, J., et al. Nat. Med., 2005, 12, 1322–9. DOI: http://doi.org/10.1038/nm1323. DOI: https://doi.org/10.1038/nm1323
Llona-Minguez, S., Baiget, J., Mackay, S.P., Pharm. Pat. Anal. 2013, 4, 481-498. DOI: https://doi.org/10.4155/ppa.13.31.
Liu, S., Misquitta, Y.R., Olland, A., Johnson, M.A., Kelleher, K.S., Kriz, R., Lin, L.L., Stahl, M., Mosyak, L. J Biol. Chem., 2013, 288, 22758-22767. https://www.rcsb.org/structure/4KIK DOI: https://doi.org/10.1074/jbc.M113.482596
Xu, G., Lo, Y.C., Li, Q., Napolitano, G., Wu, X., Jiang, X., Dreano, M., Karin, M., Wu, H., Nature, 2011, 472, 325-330. https://www.rcsb.org/structure/3rzf DOI: https://doi.org/10.1038/nature09853

Downloads
Published
Issue
Section
License
Copyright (c) 2024 Anoop Kumar Pandey, Shashwat Shukla, O. P. Yadav, Vijay Singh, Apoorva Dwivedi

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.








