Non-covalent Interactions in Dihalogenated Compounds Ch(C6H4CH2X)2 (Ch = O, S; X = Cl, Br, I). Synthesis, Crystal Structure, and Hirshfeld Surface Analysis
DOI:
https://doi.org/10.29356/jmcs.v68i2.2036Keywords:
non-covalent interactions, dihalogenated compounds, Hirshfeld surfacesAbstract
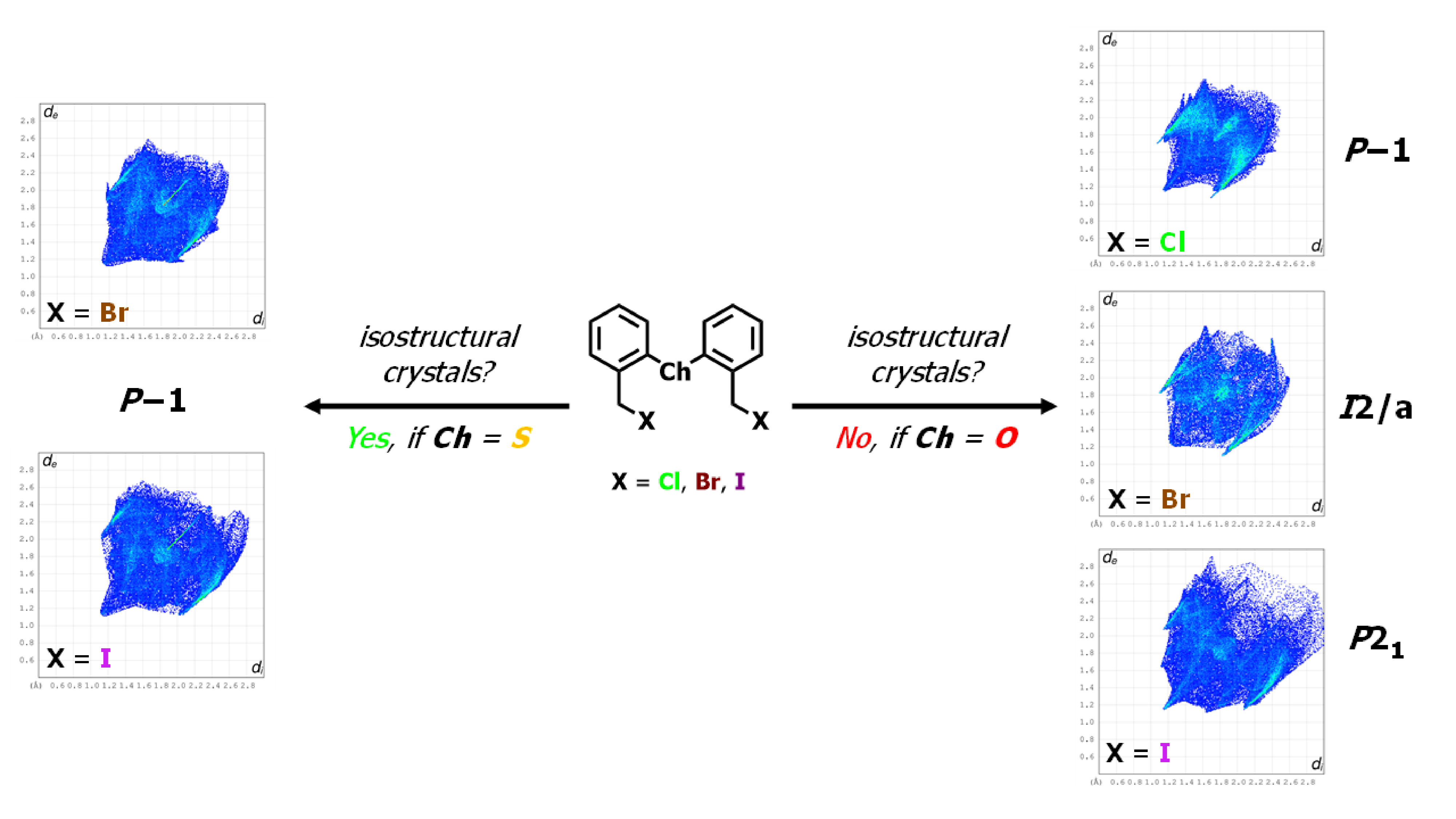
Abstract. In this work, the synthesis and structural study by means of single-crystal X-ray diffraction of compounds of general formula Ch(C6H4CH2X)2 (Ch = O, S; X = Cl, Br, I) is reported. These compounds contain two flexible hydrocarbonated arms –CH2–X in the ortho positions to the Ch heteroatom. These compounds were synthesized through a linear synthesis starting from diphenylether or diphenylsulfide. Based on the structural analysis, we describe the more relevant molecular features as well as the non-covalent interactions that the heavy halogen atoms display with other moieties that promote the cohesion of the crystal arrangement. The Hirshfeld analysis displayed that the X···π, X···X, and C–H···X interactions are quite significant in the crystal arrangement.
Resumen. En este trabajo, se describen la síntesis y el estudio estructural de difracción de rayos-X de monocristal de seis compuestos con fórmula general Ch(C6H4CH2X)2 (Ch = O, S; X = Cl, Br, I), que contienen dos brazos hidrocarbonados flexibles –CH2–X en las posiciones orto al heteroátomo Ch. Estos compuestos fueron sintetizados a través de una síntesis lineal, partiendo de difeniléter o difeniltioéter. A través del análisis estructural se describen las características moleculares más relevantes, así como las interacciones no-covalentes que presentan los átomos de halógeno pesados con otros grupos funcionales para dar cohesión a la red cristalina. El estudio de las superficies de Hirshfeld mostró que las interacciones X···π, X···X y C–H···X son muy relevantes en esta cohesión.
Downloads
References
Dean, J. A., in: Lange's Handbook of Chemistry (15th Edition), McGraw-Hill Education, New York, 1999, 330.
Häggblom, M. M.; Bossert, I. D., in: Halogenated Organic Compounds - A Global Perspective. In Dehalogenation. Springer, Boston, MA, 2004, 3-29. DOI: https://doi.org/10.1007/0-306-48011-5_1.
Patai S., Rappoport Z., in: Halides, Pseudo‐Halides and Azides; Part 1 and Part 2 in The Chemistry of Functional Groups, John Wiley & Sons Ltd, 1983, 1-223. DOI:10.1002/9780470771716. DOI: https://doi.org/10.1002/9780470771716
Anderson B. M., Meyers R. A.; in: Halogen Chemistry. In Encyclopedia of Physical Science and Technology (Third Edition), Academic Press, Elsevier, 2003, 197-222. DOI: https://doi.org/10.1016/B0-12-227410-5/00307-0.
Smolnikov, S.; Bin Shahari, M.; Dolzhenko, in: Green sustainable process for chemical and environmental engineering and science: A. Sonochemical protocols for Grignard reactions. Elsevier, 2020, 243-255. DOI: 10.1016/B978-0-12-819540-6.00009-7. DOI: https://doi.org/10.1016/B978-0-12-819540-6.00009-7
Odd Hassel – Nobel Lecture. NobelPrize.org. Nobel Prize Outreach AB 2023. Sun. 19 Mar 2023. https://www.nobelprize.org/prizes/chemistry/1969/hassel/lecture/
Desiraju, G. R.; Ho, P. S.; Kloo, L. Legon, A. C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Pure Appl. Chem. 2013, 85, 1711–1713. DOI: http://dx.doi.org/10.1351/PAC-REC-12-05-10. DOI: https://doi.org/10.1351/PAC-REC-12-05-10
Cavallo, G.; Metrangolo, P.; Milani, R.: Pilati, T.; Priimagi, A.; Resnati, G. Terraneo, G. Chem. Rev. 2016, 116, 2478-2601. DOI: https://pubs.acs.org/doi/10.1021/acs.chemrev.5b00484. DOI: https://doi.org/10.1021/acs.chemrev.5b00484
Metrangolo, P.; Neukirch, H. Pilati, T.; Resnati, G. J. Am. Chem. Soc. 2005, 38, 386–395. DOI: https://doi.org/10.1021/ar0400995.
Sutar, R. L.; Huber, S. M. J. Am. Chem. Soc. 2019, 9, 9622–9639. DOI: https://doi.org/10.1021/acscatal.9b02894.
Ding, X., Tuikka, M.; Haukka, M., in: Halogen Bonding in Crystal Engineering: Recent Advances in Crystallography, IntechOpen, 2012, 143-168. DOI: https://doi.org/10.5772/48592.
Nunzi, F.; Cesario, D.; Tarantelli, F.; Belpassi, L. Chem. Phys. Lett. 2021, 771, 138522. DOI: https://doi.org/10.1016/j.cplett.2021.138522.
Prasanna, M.D.; Guru Row, T.N. Cryst. Eng. 2000, 3, 135-154. DOI: https://doi.org/10.1016/S1463-0184(00)00035-6.
Zhao-Qi, G.; Shi-Hui, Q.; Huan-Hui, Y.; Shan-Chao, W.; Meng, Z.; Gui-Mei, T.; Yong-Tao, W.; Tao, A.; Seik-Weng N. J. Mol. Struct. 2022, 1267, 133606. DOI: https://doi.org/10.1016/j.molstruc.2022.133606.
Mejia-Rivera, F. J.; Alvarado-Rodríguez, J. G.; Andrade-López, N.; Cruz-Borbolla, J.; Jancik, V. Inorg. Chem. Commun. 2018, 97, 44-48. DOI: https://doi.org/10.1016/j.inoche.2018.09.006.
Nather, C.; Jess, I.; Kus, P.; Jones, P.G. Cryst. Eng. Comm. 2016, 18, 3142 DOI: https://doi.org/10.1039/C6CE00438E
Xu, X.; Strongin, R.M.; Fronczek, F.R. CSD Communication (Private Communication), 2015.
Oxford Diffraction CrysAlis software system, version 1.171.37.35. Oxford Diffraction Ltd., Abingdon, UK (2014)
Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann H. J. Appl. Cryst. 2009, 42, 339–341. DOI: https://doi.org/10.1107/S0021889808042726
Sheldrick, G.M. Acta Cryst. A. 2015, 71, 3–8.
Sheldrick, G.M. Acta Cryst. C. 2015, 71, 3–8. DOI: https://doi.org/10.1107/S2053229614024218
Spackman, M.A.; Jayatilaka, D. CrystEngComm. 2009, 11, 19–32 DOI: https://doi.org/10.1039/B818330A
Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. J. Appl. Cryst. 2021, 54,1006–1011. DOI: https://doi.org/10.1107/S1600576721002910
Jayatilaka, D.; Grimwood D.J. Comput. Sci. ICCS, 2003, 4, 142–151. DOI: https://doi.org/10.1007/3-540-44864-0_15
Becke A.D. J. Chem. Phys. 1993, 98, 5648–5652 DOI: https://doi.org/10.1063/1.464913
Godbout, N.; Salahub, D. R.; Andzelm J.; Wimmer Can, E. J. Chem. 1992, 70, 560–571. DOI: https://doi.org/10.1139/v92-079
Sosa, C.; Andzelm, J.; Elkin, B.C.; Wimmer, E.; Dobbs, K.D.; Dixon D.A. J. Phys. Chem. 1992, 96, 6630–6636. DOI: https://doi.org/10.1021/j100195a022
Osuka, A.; Kobayashi, F.; Maruyama, K. Bull. Chem. Soc. Jpn. 1991, 64, 1213-1225. DOI: https://doi.org/10.1246/bcsj.64.1213.
Britovsek, G. J. P.; Gibson, V. C.; Hoarau, O. D.; Spitzmesser, S. K.; White, A. J. P.; Williams, D. J. Inorg. Chem. 2003, 42, 3454-3465. DOI: https://doi.org/10.1021/ic034040. DOI: https://doi.org/10.1021/ic034040q
Martínez-Otero, D.; Alvarado-Rodríguez, J. G.; Cruz-Borbolla, J.; Andrade-López, N.; Pandiyan, T.; Moreno-Esparza, R.; Flores-Alamo, M.; Cantillo-Castillo J. Polyhedron. 2012, 33, 367-377. DOI: https://doi.org/10.1016/j.poly.2011.11.053.
Kida, T.; Kikuzawa, A.; Higashimoto, H.; Nakatsuji, Y.; Akashi, M. Tetrahedron. 2005, 61, 5763–5768. DOI: https://doi.org/10.1016/j.tet.2005.04.026.
McAdam, C.J.; Hanton, L.R.; Moratti, S.C.; Simpson, J. Acta Crystallogr. E: Crystallogr. Commun. 2015, 71, 1505-1509. DOI: 10.1107/S2056989015021295. DOI: https://doi.org/10.1107/S2056989015021295
Appel, R. Angew. Chem. Int. Ed. Engl. 1975, 14, 801-811. DOI: https://doi.org/10.1002/anie.197508011.
Cristol, S. J.; Strom, R. M.; Stull, D. P., J. Org. Chem. 1978, 43, 1150. DOI: https://doi.org/10.1021/jo00400a027.
Cordero, B.; Gómez, V.; Platero-Prats, A. E.; Revés, M.; J. Echeverría, J., Cremades, E.; Barragán F.; Alvarez, S. Dalton Trans. 2008, 21, 2832-2838. DOI: https://doi.org/10.1039/B801115J.
Gillespie, R.J. Popelier, P.L.A., in: Chemical Bonding and Molecular Geometry; From Lewis to Electron densities. Oxford University Press, New York, 2001, 84. DOI: 10.1021/ed080p31. DOI: https://doi.org/10.1021/ed080p31
Macrae, C. F.; Sovago, I.; Cottrell, S. J.; Galek, P. T. A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G. P.; Stevens, J. S.; Towler, M.; Wood, P. A. J. Appl. Cryst. 2020, 53, 226-236. DOI: https://scripts.iucr.org/cgi-bin/paper?gj5232. DOI: https://doi.org/10.1107/S1600576719014092

Downloads
Published
Issue
Section
License
Copyright (c) 2024 J. Viridiana García-González, Jose G. Alvarado-Rodríguez, Noemí Andrade-López, Cristian G. Guerra-Poot

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









