Effect of Time of Harvest on the Chemical Composition and Antioxidant Potential of Leaf Essential Oil of Syzygium guineense Growing in North Central Nigeria (Willd.) Dc. Var.
DOI:
https://doi.org/10.29356/jmcs.v68i2.2035Keywords:
Syzygium guineense, antioxidant, α-bergamotene, β-farneseneAbstract
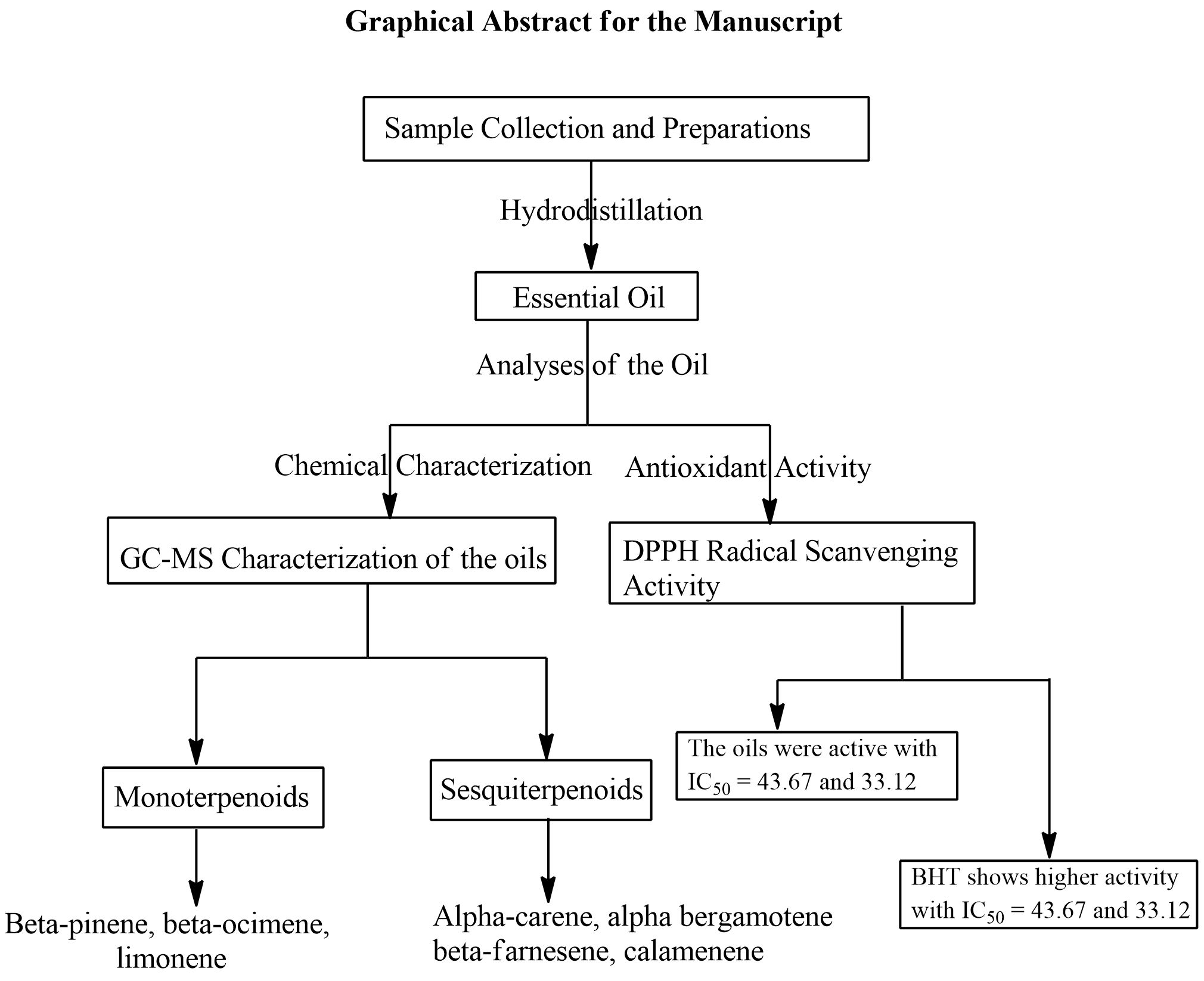
Abstract. The use of synthetic antioxidants to ameliorate oxidative stress goes with side effects. Some plants are known to be sources of natural antioxidants and, hence, could be used as alternatives to synthetic antioxidants without side effects. Meanwhile, the presence of the phytochemicals that exhibit antioxidant activity in plants depends on environmental conditions that vary with the time of harvest of plant materials. This study, therefore, investigated the effect of time of harvest on the chemical composition and antioxidant potential of leaf essential oil of Syzygium guineense native to North central Nigeria. To accomplish these, pulverized (500 g) leaves of S. guineense harvested in the morning and afternoon were separately hydrodistilled and yielded 0.25 ± 0.002 % (w/w) and 0.27 ± 0.003 % (w/w) of essential oils. Characterization of the oils using GC-MS revealed the presence of twenty-two and twenty-three compounds in the oils from morning and afternoon harvests. The most abundant compound in the oils was β-bergamotene (30.1 % and 27.3 %). D-limonene (2.9 % and 5.6 %), β-ocimene (4.2 % and 10.2 %), α-santalene (7.4 % and 7.7 %), α-cedrene (8.6 % and 9.0 %), β-farnesene (9.1 % and 10.2 %) and calamenene (7.1 % and 5.2 %) were detected in significant quantities in the oils. DPPH radial scavenging assay was used to evaluate the antioxidant activity of the oils with butylated hydroxyl toluene (BHT) as standard. The oils exhibited antioxidant activity with IC50 of 41.92 μg/mL and 33.12 μg/mL for the oils from morning and afternoon harvests. Although the oils exhibited lower antioxidant activity than the standard (IC50 of 28.63 μg/mL), but the oils could be used to ameliorate oxidative stress after clinical trials.
Resumen. El uso de antioxidantes sintéticos para mejorar el estrés oxidativo conlleva efectos secundarios. Se sabe que algunas plantas son fuentes de antioxidantes naturales y, por lo tanto, podrían usarse como alternativas a los antioxidantes sintéticos sin efectos secundarios. La presencia de fitoquímicos que exhiben la actividad antioxidante en las plantas depende de las condiciones ambientales que varían con el momento de la cosecha de los materiales vegetales. Por lo tanto, en este estudio se investigó el efecto del momento de la cosecha sobre la composición química y el potencial antioxidante del aceite esencial de hoja de Syzygium guineense, originario del centro norte de Nigeria. Para lograr esto, se hidrodestilaron por separado hojas pulverizadas (500 g) de S. guineense cosechadas en la mañana y en la tarde que produjeron 0.25 ± 0.002% (p/p) y 0.27 ± 0.003% (p/p) de aceites esenciales. La caracterización de los aceites mediante GC-MS reveló la presencia de veintidós y veintitrés compuestos en los aceites de las cosechas de la mañana y la tarde. Los compuestos más abundantes en los aceites fueron el β-bergamoteno (30.1 % y 27.3 %), D-limoneno (2.9 % y 5.6 %), β-ocimeno (4.2 % y 10.2 %), α-santaleno (7.4 % y 7.7 %), α-cedreno (8.6 % y 9.0 %), β-farneseno (9.1 % y 10.2 %) y calameneno (7.1 % y 5.2 %). Se utilizó el ensayo de eliminación radial DPPH para evaluar la actividad antioxidante de los aceites con hidroxil tolueno butilado (BHT) como estándar. Los aceites exhibieron actividad antioxidante con IC50 de 41.92 μg/mL y 33.12 μg/mL para los aceites de las cosechas de la mañana y la tarde. Si bien los aceites exhibieron una actividad antioxidante menor que el estándar (IC50 de 28.63 μg/mL), los aceites podrían usarse para mejorar el estrés oxidativo después de los ensayos clínicos.
Downloads
References
Bankefa, E.; Onileke, F.; Dada, E. J. Sci. Res. 2014, 2, 21-252.
Agwu, C. O. C.; Okeke, G. I. J. Bot. 1997, 9-10, 25–36.
Iwu, M. M. in: Handbook of African Medicinal Plants, CRP Press, BOC Raton, Florida. 1993, 12 – 78.
Burkill, H. M. in: The useful plants of west tropical families M-R, Vol. 4, 2nd Edition, Kew: Royal Botanical Gardens. 1997, 253–254.
Seyoum, G.; Abebe, M.; Asres, K.; Bekuretsion, Y.; Woldkidan, S.; Debebe, E. Evid.-based Complement. Altern. Med. 2021, 5, 1 – 10.
Wubneh, Z. B., Tadesse, S. A. BMC Complement. Altern. Med. 2017, 17, 21- 28.
Abera, B.; Adane, L.; Mamo, F. J. Pharmacogn. Phytochem. 2018, 7, 3104 – 3111.
Ezenyi, I. C.; Mbamalu, O. N.; Balogun, L.; Omorogbe, L.; Ameh, F. S.; Salawu, O. A. J. Phytopharmacol. 2016, 5, 150-156.
Kristanti, A. N.; Aung, E. A.; Aminah, N. S.; Takaya, Y.; Ramadhan, R. Open Chem. J. 2020, 18, 1256–1281.
Pieme, C. A.; Ngoupayo, J.; Nkoulou, C. H.; Moukette, B. M.; Nono, B. L.; Moor, V. J. Antioxidants. 2014, 3, 618–635.
Tadesse, A. S.; Wubneh, Z. B. BMC Complement. Altern. Med. 2017, 17, 1–7.
Okhale, S. E.; Buba, C. I.; Oladosu, P.; Ugbabe, G. E.; Ibrahim, J. A.; Egharevba, H. O.; Kunle, O. F. IJPPR. 2018, 10, 341-349.
British pharmacopoeia II. HM, stationary office, London, 1980, 109.
Adams, R. P. in Identification of essential oil components by gas chromatography and mass spectrometry. Allured publ. corp., Carol Stream, IL. USA. 2012, 78 – 109.
Jennings, W.; Shibamoto, T. in: Qualitative analysis of flavour volatiles by gas capillary chromatography. Academic press, New York, 1980; 68–109.
Joulain, D.; Koenig, W. A. in: The atlas of spectra data of sesquiterpene hydrocarbon. E. B. Verlay Hamburg, Germany, 1998, 112–153.
Usman, L. A.; Agboola, T. A.; AbdulWaheed, J. O.; Ismaeel, R. O.; Ogundele, V. A.; Ibrahim, A. NJPAS. 2016, 29, 2968–2976.
Ilhami, G. Chem-Biol. Interact. 2009, 179, 71-80.
Chalchat, J.; Noudogbessi, J.; Sohounhloue, D. C. K.; Yedomonhan, P., Figueredo, G. Rec. Nat. Prod. 2008, 2, 33-38.
Trapp, S. C.; Croteau, R. B. Genetics. 2001, 158, 811–832.
Degenhardt, J.; Ko¨llner, T. G.; Gershenzon, J. Phytochem. 2009, 70, 1621–1637.
Usman, L. A.; Watti, O. I.; Ismaeel, R. O.; Ojumoola, A. O. JOTCSA. 2016, 3, 1–18.
Iijima, Y.; Davidovich-Rikanati, R.; Fridman, E.; Gang, D. R. Plant Physiol. 2004, 136, 3724–3736.
Mkaddem, M. G.; Romdhane, M.; Ibrahim, H.; Enn-ajar, M.; Lebrihi, A.; Mathieu, F.; Bouajila, J. J. Med. Food. 2010, 13, 1500–1504.
Paolini, J.; El Ouariachi, E.; Bouyanza, A.; Tomi, P.; Hammouti, B.; Salghi, R.; Majidi, L.; Costa J. J. Med. Plants Res. 2011, 5, 5773–5778.
Hussein, A. I.; Anwar, F.; Sherazi, S. T. H.; Pryzbylski, R. Food Chem. 2008, 108, 986-995.
Boligon, A. A.; Piana, M.; Brum, T. F.; Froeder, A. L. F.; Belke, B. V.; Schwanz, T. G.; Mario, D. N.; Alves, S. H. Athayde, M. L. An. Acad. Bras. Cienc. 2014, 86, 1463-1469.
Mehta, A. A.; Shah, B. B. Asian J. Pharm. Pharmacol. 2018, 4, 883–887.
Hou, C.; Chen, Y.: Wang, C. Int. J. Food Prop. 2019. 22, 230–238.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Ajao Lamidi Usman, Ridwan Olanrewaju Ismaeel, Alfanla Kamaldeen Musa

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









