Impedance Analysis for the Study of Biofilm Formation on Electrodes: An Overview
DOI:
https://doi.org/10.29356/jmcs.v67i4.2005Keywords:
Biofilm-electrode interface, electrochemical impedance spectroscopy, electric equivalent circuit, strong electroactive biofilms, weak electroactive biofilmsAbstract
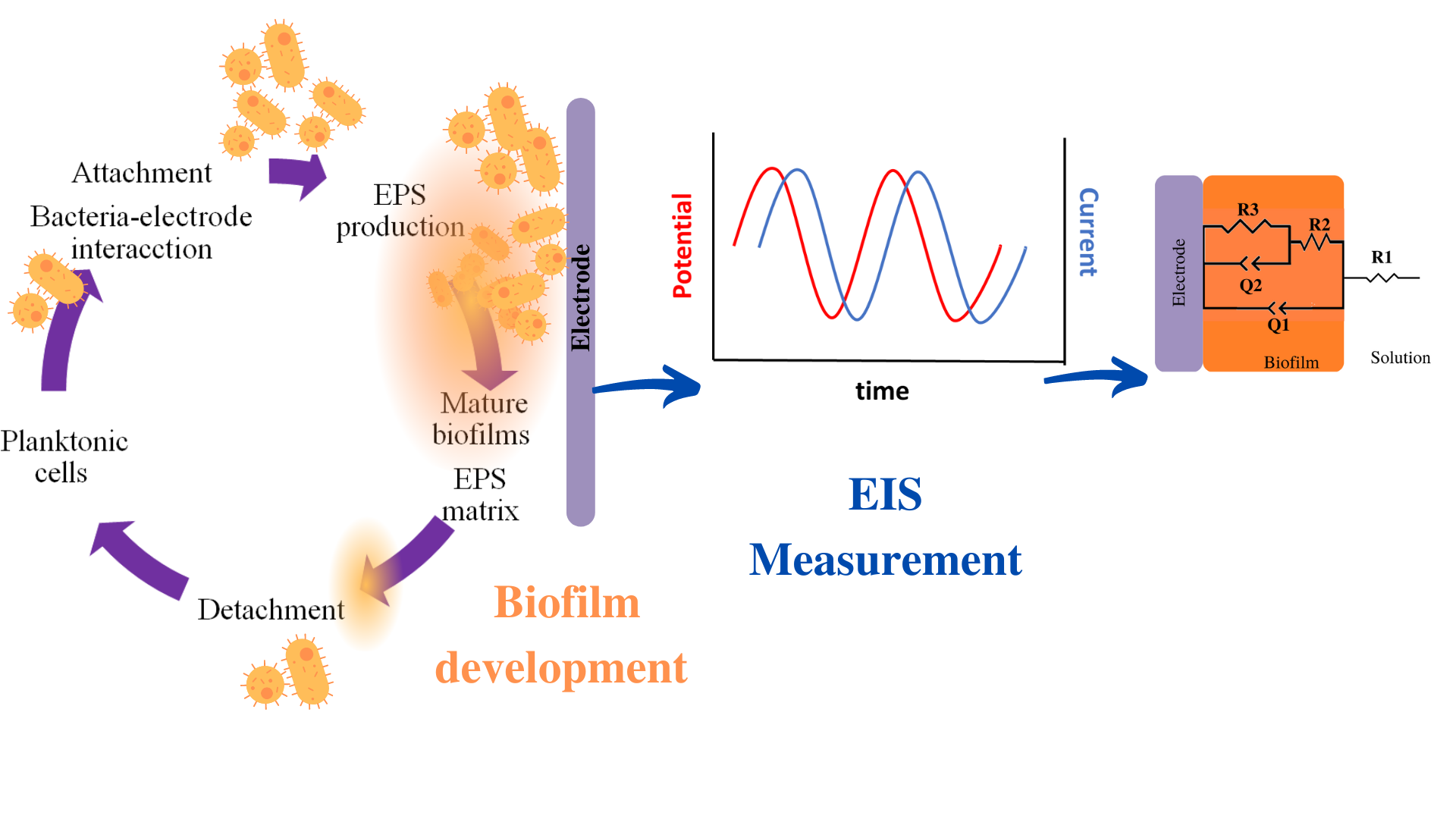
Abstract. The application of Electrochemical Impedance Spectroscopy (EIS) in biofilms studies has been long reported due to the great variety and diversity of applications that are involved in many fields, for instance, in Bioelectrochemical Systems (BES), drinking water distribution systems, electrochemical sensors, food industry, medical devices; among others. Microbial attachment and biofilm growth have been extensively investigated using EIS due to their non-destructive nature; however, several studies (using a three-electrode system) have described changes in the electrochemical parameters that model biofilm development. Therefore, this overview focused on the EIS data analysis by an electrical equivalent circuit (eec). The most representative studies on attachment, biofilm formation, Extracellular Polymeric Substances (EPS), and charge transfer phenomena were discussed.
Consequently, the goals of this overview are:
- Distinguish between the ways of studying biofilm growth (in-situ/ex-situ).
- EIS data validation by Kramers Kronig relations.
- The discussion of the electrical elements of eec.
Due to the heterogeneity of the reviewed information, the biofilms are divided into two groups: strong electroactive and another group: weak electroactive and non-electroactive biofilms. The importance of this manuscript is to present the biofilm-electrode interface by the electrical elements of various biofilms studied under different conditions, establish an overview of the working methods followed by different authors, and discuss the results obtained on diverse biofilms. Lastly, this overview might help as a general outlook for planning further research.
Resumen. La aplicación de la Espectroscopía de Impedancia Electroquímica (EIS) en estudios de biopelículas, ha sido ampliamente reportada debido a la gran variedad y diversidad de aplicaciones que están involucradas en diversos campos, por ejemplo, en sistemas bioelectroquímicos (BES), sistemas de distribución de agua potable, sensores electroquímicos, industria alimentaria, dispositivos médicos; entre otros. La adherencia microbiana y el crecimiento de biopelículas se han investigado ampliamente mediante EIS debido a su naturaleza no destructiva; sin embargo, diversos estudios han descrito cambios en los parámetros electroquímicos que modelan el desarrollo de las biopelículas. Por lo tanto, esta revisión general se centró en el análisis de datos de EIS mediante un circuito eléctrico equivalente (eec). Se discutieron los estudios más representativos sobre attachment, formación de biopelículas, Sustancias Poliméricas Extracelulares (EPS) y fenómenos de transferencia de carga. Por consiguiente, los objetivos de esta revisión son
- Distinguir entre las formas de estudiar el crecimiento de biopelículas (in-situ/ex-situ).
- La validación de los datos de EIS por relaciones de Kramers Kronig.
- La discusión de los elementos de eec.
Debido a que la información revisada sobre biopelículas es muy heterogénea, las biopelículas se dividen en dos grupos: electroactivas fuertes, y el otro grupo: electroactivas débiles y biopelículas no electroactivas.
La importancia de este manuscrito es presentar la interfase biopelícula-electrodo mediante los elementos eléctricos de varias biopelículas estudiadas bajo diferentes condiciones, establecer una visión general de los métodos de trabajo seguidos por diferentes autores y discutir los resultados obtenidos en diversos tipos de biopelículas. Por último, esta revisión contribuye como una perspectiva general para planificar futuras investigaciones.
- Distinguish between the ways of studying biofilm growth (in-situ/ex-situ)
- EIS data validation by Kramers Kronig relations
- The discussion of the electrical elements of eec
The importance of this manuscript is to present the biofilm-electrode interface by the electrical elements of various biofilms studied under different conditions, establish an overview of the working methods followed by different authors, and discuss the results obtained on diverse types of biofilms. This overview might help as a general outlook for planning further research.
Downloads
References
Ratheesh, A.; Elias, L.; Aboobakar Shibli, S. M. ACS Appl. Bio Mater. 2021, 4, 5809–5838. DOI: https://doi.org/10.1021/acsabm.1c00362.
Czerwińska-Główka, D.; Krukiewicz, K. Bioelectrochemistry. 2020, 131, 107401. DOI: https://doi.org/10.1016/j.bioelechem.2019.107401.
Donlan, R. M. Emerg. Infect. Dis. 2002, 8, 881–890. DOI: https://doi.org/10.3201/eid0809.020063.
Kim, S.; Yu, G.; Kim, T.; Shin, K.; Yoon, J. Electrochim. Acta. 2012, 82, 126–131. DOI: https://doi.org/10.1016/j.electacta.2012.05.131.
Bramhachari, P. V.; Dubey, S. K. Lett. Appl. Microbiol. 2006, 43, 571–577. DOI: https://doi.org/10.1111/j.1472-765X.2006.01967.x.
Wingender, J., Neu, T. R., Flemming, H.-C., Eds.; Springer: Berlin Heidelberg, 1999.
Seviour, T.; Derlon, N.; Dueholm, M. S.; Flemming, H.-C.; Girbal-Neuhauser, E.; Horn, H.; Kjelleberg, S.; van Loosdrecht, M. C. M.; Lotti, T.; Malpei, M. F.; et al. Water Res. 2019, 151, 1–7. DOI: https://doi.org/10.1016/j.watres.2018.11.020.
Eboigbodin, K. E.; Biggs, C. A. Biomacromolecules. 2008, 9, 686–695. DOI: https://doi.org/10.1021/bm701043c.
Jahn, A; Nielsen, P.H. Water Sci. Technol. 1995, 32. DOI: https://doi.org/10.1016/0273-1223(96)00020-0. DOI: https://doi.org/10.2166/wst.1995.0287
Ben-Yoav, H.; Freeman, A.; Sternheim, M.; Shacham-Diamand, Y. Electrochim. Acta. 2011, 56, 7780–7786. DOI: https://doi.org/10.1016/j.electacta.2010.12.025.
Borole, A. P.; Reguera, G.; Ringeisen, B.; Wang, Z.-W.; Feng, Y.; Kim, B. H. Energy Environ. Sci. 2011, 4, 4813. DOI: https://doi.org/10.1039/c1ee02511b.
Conners, E. M.; Rengasamy, K.; Bose, A. J. Ind. Microbiol. Biotechnol. 2022, 49, kuac012. DOI: https://doi.org/10.1093/jimb/kuac012.
Ter Heijne, A.; Pereira, M. A.; Pereira, J.; Sleutels, T. Trends Biotechnol. 2021, 39, 34–42. DOI: https://doi.org/10.1016/j.tibtech.2020.06.006.
Garrett, T. R.; Bhakoo, M.; Zhang, Z. Prog. Nat. Sci. 2008, 18, 1049–1056. DOI: https://doi.org/10.1016/j.pnsc.2008.04.001.
Grohmann, E.; Vaishampayan; Ahmad, I., Husain, F. M., Eds.; John Wiley & Sons, Ltd: Chichester, UK, 2017; 215–230. DOI: https://doi.org/10.1002/9781119246329.ch12.
Mizan, Md. F. R.; Bang, H.-J.; Sadekuzzaman, M.; Lee, N.; Kim, T.-J.; Ha, S.-D. Biofouling. 2017, 33, 369–378. DOI: https://doi.org/10.1080/08927014.2017.1316840.
Neu, T. R.; Manz, B.; Volke, F.; Dynes, J. J.; Hitchcock, A. P.; Lawrence, J. R. FEMS Microbiol. Ecol. 2010, 72, 1–21. DOI: https://doi.org/10.1111/j.1574-6941.2010.00837.x.
Castro, L.; Zhang, R.; Muñoz, J. A.; González, F.; Blázquez, M. L.; Sand, W.; Ballester, A. Biofouling. 2014, 30, 501–511. DOI: https://doi.org/10.1080/08927014.2014.892586.
Schlafer, S.; Meyer, R. L. J. Microbiol. Methods. 2017, 138, 50–59. DOI: https://doi.org/10.1016/j.mimet.2016.03.002.
Karimi, A.; Karig, D.; Kumar, A.; Ardekani, A. M. Lab. Chip. 2015, 15, 23–42. DOI: https://doi.org/10.1039/C4LC01095G.
Nolte, K. A.; Schwarze, J.; Rosenhahn, A. Biofouling. 2017, 33, 531–543. DOI: https://doi.org/10.1080/08927014.2017.1328058.
Furst, A. L.; Francis, M. B. Chem. Rev. 2019, 119, 700–726. DOI: https://doi.org/10.1021/acs.chemrev.8b00381.
Romero, M. C.; Ramos, G.; González, I.; Ramírez, F. Appl. Biochem. Biotechnol. 2021, 193, 1379–1396. DOI: https://doi.org/10.1007/s12010-020-03386-8.
Huerta-Miranda, G. A.; Arroyo-Escoto, A. I.; Burgos, X.; Juárez, K.; Miranda-Hernández, M. Bioelectrochemistry. 2019, 127, 145–153. DOI: https://doi.org/10.1016/j.bioelechem.2019.02.006.
Méndez-Tovar, M.; García-Meza, J. V.; González, I. Bioelectrochemistry. 2019, 128, 30–38. DOI: https://doi.org/10.1016/j.bioelechem.2019.03.004.
Drvarič Talian, S.; Moškon, J.; Dominko, R.; Gaberšček, M. Adv. Mater. Interfaces. 2022, 9, 2101116. DOI: https://doi.org/10.1002/admi.202101116.
Kretzschmar, J.; Harnisch, F. Curr. Opin. Electrochem. 2021, 29, 100757. DOI: https://doi.org/10.1016/j.coelec.2021.100757,
Logan, B. E. Wiley-Interscience: Hoboken, N.J, 2008.
Ter Heijne, A.; Schaetzle, O.; Gimenez, S.; Navarro, L.; Hamelers, B.; Fabregat-Santiago, F. Bioelectrochemistry. 2015, 106, 64–72. DOI: https://doi.org/10.1016/j.bioelechem.2015.04.002.
Heijne, A. ter; Liu, D.; Sulonen, M.; Sleutels, T.; Fabregat-Santiago, F. J. Power Sources. 2018, 400, 533–538. DOI: https://doi.org/10.1016/j.jpowsour.2018.08.003.
Piasecki, T.; Guła, G.; Nitsch, K.; Waszczuk, K.; Drulis-Kawa, Z.; Gotszalk, T. Sens. Actuators B Chem. 2013, 189, 60–65. DOI: https://doi.org/10.1016/j.snb.2012.12.087.
Agostino, V.; Ahmed, D.; Sacco, A.; Margaria, V.; Armato, C.; Quaglio, M. Electrochim. Acta. 2017, 237, 133–143. DOI: https://doi.org/10.1016/j.electacta.2017.03.186.
Lasia, A. Springer New York: New York, NY, 2014. DOI: https://doi.org/10.1007/978-1-4614-8933-7.
Trif, L.; Shaban, A.; Telegdi, J. Corros. Rev. 2018, 36, 349–363. DOI: https://doi.org/10.1515/corrrev-2017-0032.
Thapa, B. S.; Kim, T.; Pandit, S.; Song, Y. E.; Afsharian, Y. P.; Rahimnejad, M.; Kim, J. R.; Oh, S.-E. Bioresour. Technol. 2022, 347, 126579. DOI: https://doi.org/10.1016/j.biortech.2021.126579.
Sharma, M.; Bajracharya, S.; Gildemyn, S.; Patil, S. A.; Alvarez-Gallego, Y.; Pant, D.; Rabaey, K.; Dominguez-Benetton, X. Electrochim. Acta. 2014, 140, 191–208. DOI: https://doi.org/10.1016/j.electacta.2014.02.111.
Martin, A. L.; Satjaritanun, P.; Shimpalee, S.; Devivo, B. A.; Weidner, J.; Greenway, S.; Henson, J. M.; Turick, C. E. AMB Express. 2018, 8, 162. DOI: https://doi.org/10.1186/s13568-018-0692-2.
Lvovich, V. F. Wiley: Hoboken, N.J, 2012.
Orazem, M. E.; Tribollet, B. John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. DOI: https://doi.org/10.1002/9780470381588.
https://www.scribner.com/software/68-general-electrochemistr376-zview-for-windows/, accessed in September 2023.
http://www.abc.chemistry.bsu.by/vi/analyser/, accessed in March 2023.
https://jrossmacdonald.com/levmlevmw/, accessed in September 2023.
Kim, T.; Kang, J.; Lee, J.-H.; Yoon, J. Water Res. 2011, 45, 4615–4622. DOI: https://doi.org/10.1016/j.watres.2011.06.010.
Bimakr, F.; Ginige, M. P.; Kaksonen, A. H.; Sutton, D. C.; Puzon, G. J.; Cheng, K. Y. Sens. Actuators B Chem. 2018, 277, 526–534. DOI: https://doi.org/10.1016/j.snb.2018.09.005.
Marsili, E.; Rollefson, J. B.; Baron, D. B.; Hozalski, R. M.; Bond, D. R. Appl. Environ. Microbiol. 2008, 74, 7329–7337. DOI: https://doi.org/10.1128/AEM.00177-08.
Babauta, J. T.; Beyenal, H. Biotechnol. Bioeng. 2014, 111, 285–294. DOI: https://doi.org/10.1002/bit.25105.
Wolf-Baca, M.; Grzebyk, T.; Siedlecka, A. Int. J. Environ. Res. 2022, 16, 64. DOI: https://doi.org/10.1007/s41742-022-00438-1.
Mira, A.; Buetas, E.; Rosier, B. T.; Mazurel, D.; Villanueva-Castellote, Á.; Llena, C.; Ferrer, M. D. J. Oral Microbiol. 2019, 11, 1609838. DOI: https://doi.org/10.1080/20002297.2019.1609838.
Illig, J.; Ender, M.; Chrobak, T.; Schmidt, J. P.; Klotz, D.; Ivers-Tiffée, E. J. Electrochem. Soc. 2012, 159, A952–A960. DOI: https://doi.org/10.1149/2.030207jes.
Macdonald, D. D.; Urquidi‐Macdonald, M. J. Electrochem. Soc. 1985, 132, 2316–2319. DOI: https://doi.org/10.1149/1.2113570.
Urquidi-Macdonald, M.; Real, S.; Macdonald, D. D. Electrochim. Acta. 1990, 35, 1559–1566. DOI: https://doi.org/10.1016/0013-4686(90)80010-L.
Boukamp, B. A. J. Electrochem. Soc. 1995, 142, 1885–1894. DOI: https://doi.org/10.1149/1.2044210.
Elsøe, K.; Kraglund, M. R.; Grahl-Madsen, L.; Scherer, G. G.; Hjelm, J.; Jensen, S. H.; Jacobsen, T.; Mogensen, M. B. Fuel Cells. 2018, 18, 640–648. DOI: https://doi.org/10.1002/fuce.201800005.
Grdeń, M. J. Solid State Electrochem. 2017, 21, 3333–3344. DOI: https://doi.org/10.1007/s10008-017-3684-2.
Schönleber, M.; Klotz, D.; Ivers-Tiffée, E. Electrochim. Acta. 2014, 131, 20–27. DOI: https://doi.org/10.1016/j.electacta.2014.01.034.
Esteban, J. M.; Orazem, M. E. J. Electrochem. Soc. 1991, 138, 67–76. DOI: https://doi.org/10.1149/1.2085580.
Boigues Muñoz, C.; Pumiglia, D.; McPhail, S. J.; Montinaro, D.; Comodi, G.; Santori, G.; Carlini, M.; Polonara, F. J. Power Sources. 2015, 294, 658–668. DOI: https://doi.org/10.1016/j.jpowsour.2015.06.118.
https://www.iam.kit.edu/et/english/Lin-KK.php, accessed in September 2023.
Varshney, M.; Li, Y. Talanta. 2008, 74, 518–525. DOI: https://doi.org/10.1016/j.talanta.2007.06.027.
Doyle, L. E.; Marsili, E. Bioresour. Technol. 2018, 258, 354–364. DOI: https://doi.org/10.1016/j.biortech.2018.02.073.
Castellano-Hinojosa, A.; González-Martínez, A.; Pozo, C.; González-López, J. J. Water Process Eng. 2022, 50, 103199. DOI: https://doi.org/10.1016/j.jwpe.2022.103199.
Logan, B. E.; Rossi, R.; Ragab, A.; Saikaly, P. E. Nat. Rev. Microbiol. 2019, 17, 307–319. DOI: https://doi.org/10.1038/s41579-019-0173-x.
Li, H.; Liao, B.; Xiong, J.; Zhou, X.; Zhi, H.; Liu, X.; Li, X.; Li, W. J. Power Sources. 2018, 379, 115–122. DOI: https://doi.org/10.1016/j.jpowsour.2018.01.040.
Aiyer, K.; Doyle, L. E. Trends Biotechnol. 2022, 40, 564–575. DOI: https://doi.org/10.1016/j.tibtech.2021.10.002.
Wang, V. B.; Chua, S.-L.; Cao, B.; Seviour, T.; Nesatyy, V. J.; Marsili, E.; Kjelleberg, S.; Givskov, M.; Tolker-Nielsen, T.; Song, H.; et al. PLoS ONE. 2013, 8, e63129. DOI: https://doi.org/10.1371/journal.pone.0063129.
Wright, J.; Moreland, P.; Wipat, A.; Zhang, M.; Dade-Robertson, M. Access Microbiol. 2020, 2. DOI: https://doi.org/10.1099/acmi.ac2020.po0143.
Eghtesadi, N.; Olaifa, K.; Perna, F. M.; Capriati, V.; Trotta, M.; Ajunwa, O.; Marsili, E. Bioelectrochemistry. 2022, 147, 108207. DOI: https://doi.org/10.1016/j.bioelechem.2022.108207.
Kondaveeti, S.; Lee, S.-H.; Park, H.-D.; Min, B. Electrochim. Acta. 2020, 331, 135388. DOI: https://doi.org/10.1016/j.electacta.2019.135388.
Sun, D.; Chen, J.; Huang, H.; Liu, W.; Ye, Y.; Cheng, S. Int. J. Hydrog. Energy. 2016, 41, 16523–16528. DOI: https://doi.org/10.1016/j.ijhydene.2016.04.163.
Pandit, S.; Khilari, S.; Roy, S.; Pradhan, D.; Das, D. Bioresour. Technol. 2014, 166, 451–457. DOI: https://doi.org/10.1016/j.biortech.2014.05.075.
Danese, P. N.; Pratt, L. A.; Kolter, R. J. Bacteriol. 2000, 182, 3593–3596. DOI: https://doi.org/10.1128/JB.182.12.3593-3596.2000.
Reisner, A.; Haagensen, J. A. J.; Schembri, M. A.; Zechner, E. L.; Molin, S. Mol. Microbiol. 2003, 48, 933–946. DOI: https://doi.org/10.1046/j.1365-2958.2003.03490.x.
Vidal, O.; Longin, R.; Prigent-Combaret, C.; Dorel, C.; Hooreman, M.; Lejeune, P. J. Bacteriol. 1998, 180, 2442–2449. DOI: https://doi.org/10.1128/JB.180.9.2442-2449.1998.
Maurício, R.; Dias, C. J.; Santana, F. Environ. Monit. Assess. 2006, 119, 599–607. DOI: https://doi.org/10.1007/s10661-005-9045-0.
Colvin, K. M.; Irie, Y.; Tart, C. S.; Urbano, R.; Whitney, J. C.; Ryder, C.; Howell, P. L.; Wozniak, D. J.; Parsek, M. R. Environ. Microbiol. 2012, 14, 1913–1928. DOI: https://doi.org/10.1111/j.1462-2920.2011.02657.x.
Marvasi, M.; Visscher, P. T.; Casillas Martinez, L. FEMS Microbiol. Lett. 2010, 313, 1–9. DOI: https://doi.org/10.1111/j.1574-6968.2010.02085.x.
Harimawan, A.; Ting, Y.-P. Colloids Surf. B Biointerfaces. 2016, 146, 459–467. DOI: https://doi.org/10.1016/j.colsurfb.2016.06.039.
Marsili, E.; Sun, J.; Bond, D. R. Electroanalysis. 2010, 22, 865–874. DOI: https://doi.org/10.1002/elan.200800007.
Srikanth, S.; Marsili, E.; Flickinger, M. C.; Bond, D. R. Biotechnol. Bioeng. 2008, 99, 1065–1073. DOI: https://doi.org/10.1002/bit.21671.
Babauta, J. T.; Beyenal, H. J. Power Sources. 2017, 356, 549–555. DOI: https://doi.org/10.1016/j.jpowsour.2017.03.021.
Stöckl, M.; Teubner, N. C.; Holtmann, D.; Mangold, K.-M.; Sand, W. ACS Appl. Mater. Interfaces. 2019, 11, 8961–8968. DOI: https://doi.org/10.1021/acsami.8b14340.
Yang, G.; Lin, J.; Zeng, E. Y.; Zhuang, L. Bioresour. Technol. 2019, 276, 119–126. DOI: https://doi.org/10.1016/j.biortech.2018.12.100.
Fernández-Reyes, J. S.; García-Meza, J. V. Biotechnol. Lett. 2018, 40, 63–73. DOI: https://doi.org/10.1007/s10529-017-2435-x.
Paredes, J.; Becerro, S.; Arizti, F.; Aguinaga, A.; Del Pozo, J. L.; Arana, S. Biosens. Bioelectron. 2012, 38, 226–232. DOI: https://doi.org/10.1016/j.bios.2012.05.027.
Yang, L.; Li, Y. J. Microbiol. Methods. 2006, 64, 9–16. DOI: https://doi.org/10.1016/j.mimet.2005.04.022.
Song, J.; Li, Y.; Yin, F.; Zhang, Z.; Ke, D.; Wang, D.; Yuan, Q.; Zhang, X.-E. ACS Sens. 2020, 5, 1795–1803. DOI: https://doi.org/10.1021/acssensors.0c00570.

Downloads
Published
Issue
Section
License
Copyright (c) 2023 María Concepcion Romero Serrano, Marcela Mendez Tovar

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









