Evaluation of Photosensitizing Ability of Antioxidants Used in Skincare Products
DOI:
https://doi.org/10.29356/jmcs.v68i2.1925Keywords:
antioxidants, singlet oxygen, photosensitizing ability, photo-oxidation of ergosterolAbstract
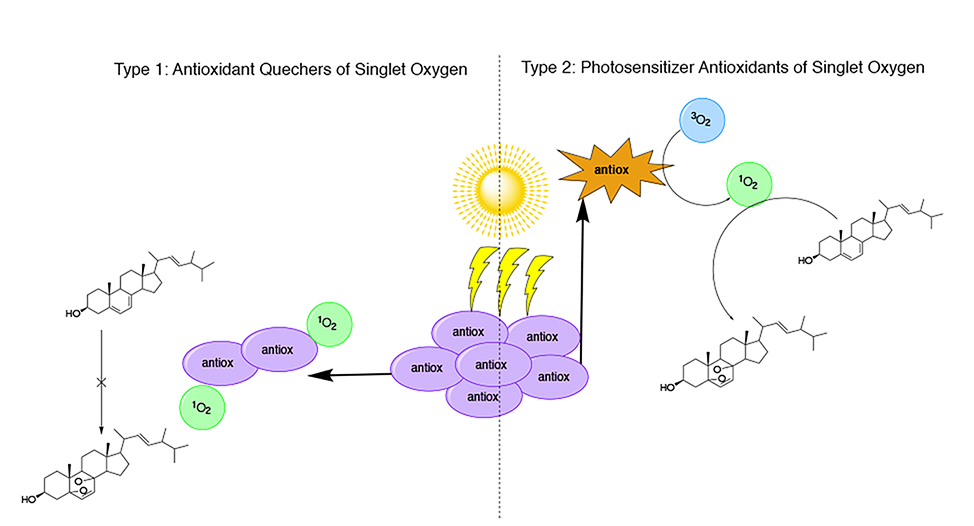
Abstract. Singlet oxygen generation is possible by photosensitizer molecules able to absorb energy from light and transfer it to molecular oxygen. Singlet oxygen is able to react with components of cellular membranes such as cholesterol leading to peroxidation products implicated in photoaging. In order to prevent oxidative damage caused by reactive oxygen species, skincare products enriched with antioxidants have been developed; in spite of some pro-oxidant effects associated with antioxidants has been reported. Based on this data, the photosensitizing ability of 14 antioxidants commonly used in skincare products was evaluated through the photo-oxidation of ergosterol, using ergosterol as oxidizable substrate to quench singlet oxygen. Singlet oxygen indirectly detection was performed through 1H-NMR mixtures analysis by ergosterol peroxide detection. The results revealed that fisetin, retinol, cyanidin and hesperetin they acted as photosensitizer antioxidants in generation of singlet oxygen. Conversely, caffeic acid, luteolin, rutin, vanillic acid, ascorbic acid, apigenin, epigallocatechin gallate, rosmarinic acid, myricetin and kaempferol were not able to generate singlet oxygen through a photosensitized mechanism. Our results allow us to suggest that the incorporation of antioxidants in skincare products as anti-aging treatments should be supported by their evaluation against photosensitizing ability in order to increase their safety.
Resumen. La generación del oxígeno singulete es posible a través de moléculas fotosensibilizadoras capaces de absorber energía proveniente de la luz y transferirla al oxígeno molecular. El oxígeno singulete es capaz de reaccionar con componentes de membranas celulares como el colesterol formando productos de peroxidación implicados en el foto-envejecimiento. Para prevenir el daño oxidativo causado por especies reactivas del oxígeno, se han desarrollado productos para el cuidado de la piel enriquecidos con antioxidantes, a pesar de que han sido reportados algunos efectos prooxidantes asociados a los antioxidantes. Con base en lo anterior, se evaluó la capacidad fotosensibilizadora de 14 antioxidantes comúnmente utilizados en productos para el cuidado de la piel mediante la foto-oxidación de ergosterol, utilizando ergosterol como sustrato oxidable para atrapar oxígeno singulete. La detección indirecta del oxígeno singulete se realizó mediante análisis de mezclas de RMN-1H a través de la detección de peróxido de ergosterol. Los resultados mostraron que fisetina, retinol, cianidina y hesperetina actuaron como antioxidantes fotosensibilizadores en la generación de oxígeno singulete. Por el contrario, ácido cafeico, luteolina, rutina, ácido vainillínico, ácido ascórbico, apigenina, galato de epigalocatequina, ácido rosmarínico, miricetina y kaempferol no fueron capaces de generar oxígeno singulete mediante mecanismos fotosensibilizados. Los resultados permiten sugerir que la incorporación de antioxidantes en productos para el cuidado de la piel como tratamiento anti-envejecimiento debe respaldarse con la evaluación de la capacidad fotosensibilizadora para incrementar su seguridad.
Downloads
References
Onyango, A. N. Oxid. Med. Cell. Longev. 2016, 2016, 1–22. DOI: https://doi.org/10.1155/2016/2398573.
Miyamoto, S.; Martinez, G. R.; Medeiros, M. H. G.; Di, P. J. Photochem. Photobiol. B. 2014, 139, 24–33. DOI: https://doi.org/10.1016/j.jphotobiol.2014.03.028.
Akhalaya, M. Y.; Maksimov, G. V.; Rubin, A. B.; Lademann, J.; Darvin, M. E. Ageing Res. Rev. 2014, 16, 1–11. DOI: https://doi.org/10.1016/j.arr.2014.03.006.
Calori, I. R.; Gusmão, L. A.; Tedesco, A. C. J. Photochem. Photobiol. 2021, 7, 100041. DOI: https://doi.org/10.1016/j.jpap.2021.100041.
Baptista, M. S.; Cadet, J.; Di Mascio, P.; Ghogare, A. A.; Greer, A.; Hamblin, M. R.; Lorente, C.; Nunez, S. C.; Ribeiro, M. S.; Thomas, A. H.; Vignoni, M.; Yoshimura, T. M. Photochem. Photobiol. 2017, 93, 912–919. DOI: https://doi.org/10.1111/php.12716.
Lorente, C.; Serrano, M. P.; Vignoni, M.; Dántola, M. L.; Thomas, A. H. J. Photochem. Photobiol. 2021, 7, 100045. DOI: https://doi.org/10.1016/J.JPAP.2021.100045.
Freitas, J. V.; Praça, F. S. G.; Bentley, M. V. L. B.; Gaspar, L. R. Int. J. Pharm. 2015, 484, 131–137. DOI: https://doi.org/10.1016/j.ijpharm.2015.02.062.
Pentek, T.; Newenhouse, E.; O’Brien, B.; Singh Chauhan, A. Molecules. 2017, 22, 1–16. DOI: https://doi.org/10.3390/molecules22010137.
Hoang, H. T.; Moon, J. Y.; Lee, Y. C. Cosmetics. 2021, 8, 106. DOI: https://doi.org/10.3390/COSMETICS8040106.
Halliwell, B.; Gutteridge, J. M. C. Free Radicals in Biology and Medicine, Illustrate.; Halliwell, B., Gutteridge, J. M. C., Eds.; Oxford University Press: New York, 2015.
Pisoschi, A. M.; Pop, A. Eur. J. Med. Chem. 2015, 97, 55–74. DOI: https://doi.org/10.1016/j.ejmech.2015.04.040.
Fahlman, B. M.; Krol, E. S. J. Photochem. Photobiol. B. 2009, 97, 123–131. DOI: https://doi.org/10.1016/j.jphotobiol.2009.08.009.
Celaje, J. A.; Zhang, D.; Guerrero, A. M.; Selke, M. Org. Lett. 2011, 13, 4846–4849. DOI: https://doi.org/10.1021/ol201922u.
Rajnochová Svobodová, A.; Ryšavá, A.; Psotová, M.; Kosina, P.; Zálešák, B.; Ulrichová, J.; Vostálová, J. Photochem. Photobiol. 2017, 93, 1240–1247. DOI: https://doi.org/10.1111/php.12755.
Lagunes, I.; Trigos, Á. J. Photochem. Photobiol. B. 2015, 145, 30–34. DOI: https://doi.org/10.1016/j.jphotobiol.2015.02.014.
Vázquez-Ortega, F.; Lagunes, I.; Trigos, Á. Dyes Pigm. 2020, 176, 108248. DOI: https://doi.org/10.1016/j.dyepig.2020.108248.
Vázquez-Ortega, F.; Sifaoui, I.; Reyes-Batlle, M.; Piñero, J. E.; Lagunes, I.; Trigos, Á.; Lorenzo-Morales, J.; Díaz-Marrero, A. R.; Fernández, J. J. Dyes Pigm. 2020, 180, 108481. DOI: https://doi.org/10.1016/j.dyepig.2020.108481.
Guerrero, T.; Vázquez-Ortega, F.; Lagunes, I.; Ortiz-Blanco, E.; Sosa-Ortiz, G.; Tovar-Miranda, R.; Medina, M. E.; Trigos, Á. Dyes Pigm. 2021, 192, 109447. DOI: https://doi.org/10.1016/J.DYEPIG.2021.109447.
Yin, H.; Xu, L.; Porter, N. A. Chem. Rev. 2011, 111, 5944–5972. DOI: https://doi.org/10.1021/cr200084z.
Minami, Y.; Kawabata, K.; Kubo, Y.; Arase, S.; Hirasaka, K.; Nikawa, T.; Bando, N.; Kawai, Y.; Terao, J. J. Nutr. Biochem. 2009, 20, 389–398. DOI: https://doi.org/10.1016/j.jnutbio.2008.04.010.
Seo, S. H.; Jeong, G. S. Int. Immunopharmacol. 2015, 29, 246–253. DOI: https://doi.org/10.1016/j.intimp.2015.11.014.
Chiang, H. M.; Chan, S. Y.; Chu, Y.; Wen, K. C. J. Agric. Food Chem. 2015, 63, 4551–4560. DOI: https://doi.org/10.1021/jf502500t.
Shao, Y.; He, T.; Fisher, G. J.; Voorhees, J. J.; Quan, T. Int. J. Cosmet. Sci. 2017, 39, 56–65. DOI: https://doi.org/10.1111/ics.12348.
Pratheeshkumar, P.; Son, Y. O.; Wang, X.; Divya, S. P.; Joseph, B.; Hitron, J. A.; Wang, L.; Kim, D.; Yin, Y.; Roy, R. V.; Lu, J.; Zhang, Z.; Wang, Y.; Shi, X. Toxicol. Appl. Pharmacol. 2014, 280, 127–137. DOI: https://doi.org/10.1016/j.taap.2014.06.028.
Li, M.; Lin, X. F.; Lu, J.; Zhou, B. R.; Luo, D. J. Photochem. Photobiol. B. 2016, 165, 240–245. DOI: https://doi.org/10.1016/j.jphotobiol.2016.10.037.
Choi, K. S.; Kundu, J. K.; Chun, K. S.; Na, H. K.; Surh, Y. J. Arch. Biochem. Biophys. 2014, 559, 38–45. DOI: https://doi.org/10.1016/j.abb.2014.05.016.
Lembo, S.; Balato, A.; di Caprio, R.; Cirillo, T.; Giannini, V.; Gasparri, F.; Monfrecola, G. Biomed. Res. Int. 2014, 2014, 346793. DOI: https://doi.org/10.1155/2014/346793.
Choi, S.; Youn, J.; Kim, K.; Joo, D. H.; Shin, S.; Lee, J.; Lee, H. K.; An, I.; Kwon, S.; Youn, H.J.; Ahn, K. J.; An, S.; Cha, H. J. Int. J. Mol. Med. 2016, 38, 627–634. DOI: https://doi.org/10.3892/ijmm.2016.2626.
Chen, J.; Li, Y.; Zhu, Q.; Li, T.; Lu, H.; Wei, N.; Huang, Y.; Shi, R.; Ma, X.; Wang, X.; Sheng, J. Mech. Ageing. Dev. 2017, 164, 1–7. DOI: https://doi.org/10.1016/j.mad.2017.03.007.
Xie, J.; Zheng, Y. J. Cosmet. Dermatol. 2017, 16, 444–449. DOI: https://doi.org/10.1111/jocd.12399.
Aguiar, B.; Carmo, H.; Garrido, J.; Lobo, J. M. S.; Almeida, I. F. Molecules. 2022, 27, 189. DOI: https://doi.org/10.3390/MOLECULES27010189.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Irene Lagunes, Ángel Trigos

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









