Kinetic Study of Ru(III) Promoted Oxidation of L-Tryptophan in an Anionic Surfactant Medium by Hexacyanoferrate(III)
DOI:
https://doi.org/10.29356/jmcs.v67i1.1829Keywords:
Critical micellar concentration, Surfactant, L-tryptophan, Activation parameters, Kinetics and Mechanism, Hexacyanoferrate(III)Abstract
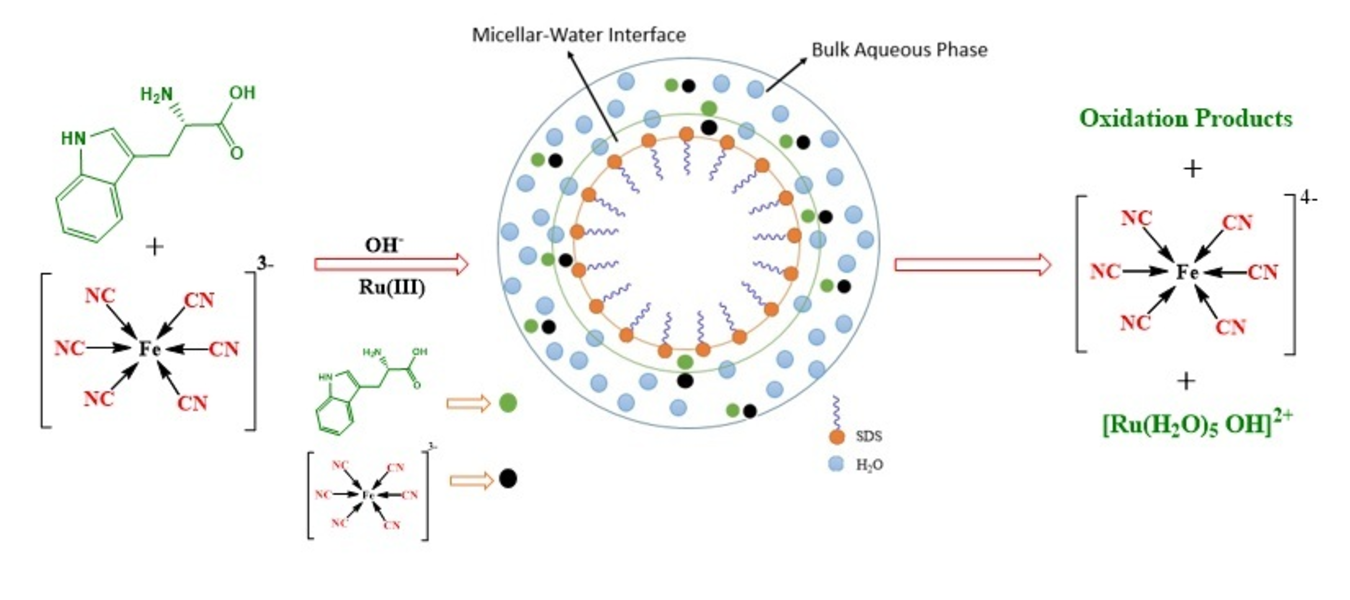
The kinetic investigation of Ru(III) promoted oxidation of L-tryptophan (Trp) by [Fe(CN)6]3- has been performed in anionic sodium dodecyl sulfate (SDS) micellar medium by recording the decrease in absorbance at 420 nm, corresponding to [Fe(CN)6]3- using UV-visible spectrophotometer. Pseudo-first-order condition has been used to examine the progress of reaction as a function of [Fe(CN)63−], ionic strength, [OH-], [SDS], [Ru3+], [Trp], and temperature by changing one variable at a time. The results exhibit that [OH-], [SDS], and [Trp] are the decisive parameter showing an appreciable effect on reaction rate. The reaction exhibits first-order kinetics in the studies concentration range of Ru(III), [Fe(CN)6]3− and at lower [Trp] and [OH-]. The incremental trend observed in the reaction rate with electrolyte concentration shows a positive salt effect. The reaction rate is almost ten times faster in SDS micellar medium compared to the aqueous medium. [Fe(CN)6]3- does not show any appreciable effect on the critical micellar concentration (CMC) of SDS as the polar head of SDS and [Fe(CN)6]3- both are negatively charged. The K+ obtained from K3[Fe(CN)6] and KNO3 decreases the repulsion between the negatively charged heads of the surfactant molecules thereby decreasing the CMC of SDS. The activation parameters also support the outer-sphere electron transfer mechanism as proposed by us.
Resumen. El estudio cinético de la oxidación de L-tryptofano (Trp) con [Fe(CN)6]3- asistida por Ru(III), se llevó a cabo en un medio micelar de dodecilsulfato de sodio aniónico (SDS) y se monitoreó utilizando espectrometría de UV-visible midiendo la disminución de la absorbancia a 420 nm, correspondiente al [Fe(CN)6]3-. Para examinar el avance de la reaccción se utilizaron condiciones de pseudo-primer orden en función de [Fe(CN)63−], fuerza iónica, [OH-], [SDS], [Ru3+], [Trp], y temperatura, variando siempre una sola una variable. Los resultados indican, que los parametros decisivos que tuvieron un efecto apreciable sobre la velocidad de la reacción son [OH-], [SDS], y [Trp]. La reacción sigue una cinética de primer orden en el rango de concentraciones de estudio de Ru(III), [Fe(CN)6]3− y a bajas concentraciones de [Trp] y [OH-]. La tendencia de incremento de velocidad de la reacción con aumento de la concentración del electrolito muestra un efecto salino positivo. La velocidad de la reacción en el medio micelar de SDS es casi diez veces mayor que en solución acuosa. [Fe(CN)6]3- no muestra ningún efecto appreciable en la concentración crítica micelar (CMC) de SDS debido a que el grupo polar del SDS (SO3-, cabeza) y el [Fe(CN)6]3- tienen ambos carga negativa. Los cationes K+ provenientes del K3[Fe(CN)6] y KNO3 disminuyen la repulsión entre las cabezas con cargas negativas del surfactante, bajando así la CMC del SDS. Los parámetros de activación apoyan también el mecanismo de transferencia de electrones de la esfera exterior propuesto.
Downloads
References
Das, B.; Kumar, B.; Begum, W. Chem. Afr. 2022, DOI: https://doi.org/10.1007/s42250-022-0345-0
Zahed, M.A.; Matinvafa, M.A.; Azari, A. Discov. Water 2022, 2, 5. DOI: https://doi.org/10.1007/s43832-022-00013-x
Chinnaraja, M.D.; Dsouza, J. SPAST Abstracts 2021, 1.
Karimi, M.A.; Mozaheb, M.A.; Hatefi-Mehrjardi, A. J. Anal. Sci. Technol. 2015, 6, 1-8. DOI: https://doi.org/10.1186/s40543-015-0077-y
Shah, S.; Chatterjee, S.K.; Bhattarai, A. J. Surfactants Deterg. 2016, 19, 201-207. DOI: https://doi.org/10.1007/s11743-015-1755-x
Motin, A.; Hafiz Mia, M.A.; Nasimul Islam, A.K.M. J. Saudi. Chem. Soc. 2015, 19, 172-180. DOI: https://doi.org/10.1016/j.jscs.2012.01.009
Mukerjee, P. J. Phys. Chem. 1972, 76, 565-570. DOI: https://doi.org/10.1021/j100648a019
Muller, N. J. Phys. Chem. 1972, 76, 3017-3020. DOI: https://doi.org/10.1021/j100665a017
Muller, N. J. Phys. Chem. 1975, 79, 287-291. DOI: https://doi.org/10.1021/j100570a019
Oakenfull, D.G.; Fendwich, D.E. J. Phys. Chem. 1974, 78, 1759-1763. DOI: https://doi.org/10.1021/j100610a018
Tanford, C. J. Phys. Chem. 1972, 76, 3020-3024. DOI: https://doi.org/10.1021/j100665a018
Paula, S.; Sues, W.; Tuchtenhagen, J.; Blume, A. J. Phys. Chem. 1995, 99, 11742-11751. DOI: https://doi.org/10.1021/J100030A019
Iioka, T.; Takahashi, S.; Yoshida, Y.; Matsumura, Y.; Hiraoka, S.; Sato, H.J. Comput. Chem. 2019, 40, 279-285. DOI: https://doi.org/10.1002/jcc.25588
Naik, R.M.; Srivastava, A.; Tiwari, A.K.; Yaday, S.B.S.; Verma, A.K. J. Iran. Chem. Soc. 2007, 4, 63–71. DOI: https://doi.org/10.1007/bf03245804
Naik, R.M.; Srivastava, A.; Verma, A K.; Yadav, S.B.S.; Singh, R.; Prasad, S. Bioinorg. Reac. Mech. 2007, 6, 185-192. DOI: https://doi.org/10.1515/irm.2007.6.3.185
Omondi, R.O.; Stephen, O.; Ojwach, S.O.; Jaganyi, D. Inorg. Chim. Acta. 2020, 512, 119883. DOI: https://doi.org/10.1016/j.ica.2020.119883
Sharma, V. Biointerface Res. App. Chem. 2022, 12, 7064–7074. DOI: https://doi.org/10.33263/briac125.70647074
Sharma, V.; Vashistha, V.K.; Das, D.K. Biointerface Res. App. Chem. 2020, 11, 7393-7399. DOI: https://doi.org/10.33263/briac111.73937399
Srivastava, A.; Naik, R.M.; Rai, J.; Asthana, A. Russ. J. Phy. Chem. 2021, 95, 2545–2552; DOI: https://doi.org/10.1134/s0036024421130227
Singh, A.; Singh, A. Prog. Reac. Kinet. Mech. 2013, 38, 105-118. DOI: https://doi.org/10.3184/146867813x13600909461700
Naik, R.M.; Tewari, R.K.; Singh, P.K.; Tiwari, A.K.; Prasad, S. Trans. Met. Chem. 2005, 30, 968–977. DOI: https://doi.org/10.1007/s11243-005-6266-6
Prasad, S.; Naik, R.M.; Srivastava, A. Spectrochim. Acta A. 2008, 70, 958-965. DOI: https://doi.org/10.1016/j.saa.2007.10.011
Srivastava, A.; Sharma, V.; Prajapati, A.; Srivastava, N.; Naik, R.M. Chem. Chem. Technol. 2019, 13, 275-279. DOI: https://doi.org/10.23939/chcht13.03.275
Srivastava, A.; Sharma, V.; Singh, V.K.; Srivastava, K. J. Mex. Chem. Soc. 2022, 66, 57-69. DOI: https://doi.org/10.29356/jmcs.v66i1.1654
Gupta, D.; Bhardwaj, S., Sethi, S., Pramanik, S.; Das, D.K.; Kumar, R.; Singh, P.P.; Vashistha, V.K. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2022, 270, 120819. DOI: https://doi.org/10.1016/j.saa.2021.120819
Vashistha, V.K.; Bhushan, R. Biomed. Chromatogr. 2019, 33, e4550. DOI: https://doi.org/10.1002/bmc.4550
Agrawal, G.P.; Maheshwari, R.K.; Mishra, P. Curr. Pharm. Anal. 2020, 16, 487-493. DOI: https://doi.org/10.2174/1573412914666181024145937
Gupta, A.; Pey, A. Ind. J. Sci. Res. 2017, 13, 66-72.
Asghar, B.H.; Altas, H.M.; Fawzi, A. J. Sau. Chem. Soc. 2007, 21, 887-898. DOI: https://doi.org/10.1016/j.jscs.2015.12.003
Pey, E.; Grover, N.; Kambo, N.; Uphadyay, S.K. Ind. J Chem. 2004, 43A, 1183-1192.
Naik, R.M.; Srivastava, A.; Verma, A.K. Turk. J. Chem. 2008, 32, 495-503. DOI: https://doi.org/10.3906/che-0612-7
Sharanabasamma, K.; Angadi, M.A.; Tuwar, S.M. Open Catal. J. 2011, 4, 1-8. DOI: https://doi.org/10.2174/1876214x01104010001
Singh, H.S.; Singh, B.; Gupta, A.; Singh, A.K. Oxid Commun. 1999, 22, 146-153.
Goel, A.; Sharma, R. J. Chem. Eng. Mater. Sci. 2012, 3, 1–6. DOI: https://doi.org/10.5897/jcems11.049
Nowdari, A.; Adari, K.K.; Gollapalli, N.R.; Parvataneni, V.E. J. Chem. 2009, 6, 93–98. doi: https://doi.org/10.1155/2009/615750
Goel, A.; Sharma, S. Trans. Met. Chem. 2010, 35, 549–554. DOI: https://doi.org/10.1007/s11243-010-9362-1
37.Bagalkoti, J.; Nibewoor, S.T. Monatsh. Chem. 2019, 150, 1469–1478. DOI: https://doi.org/10.1007/s00706-019-02482-8
Mukherjee, K.; Saha, B.J. Korean Chem. Soc. 2013, 57, 425-431. DOI: https://doi.org/10.5012/jkcs.2013.57.4.425
Zouraba, S.M.; Ezzob, E.M.; El-Ailac, H.J.; Salema, J.K.J. J. Surfactants Deterg. 2005, 8, 83-89. DOI: https://doi.org/10.1007/s11743-005-0334-6
Rastogi, R.; Srivastava, A.; Naik, R.M. J. Disp. Sci. Tech. 2020, 41, 1045-1050. DOI: https://doi.org/10.1080/01932691.2019.1614042
Srivastava, A.; Naik, R.M.; Rastogi, R. J. Iran. Chem. Soc. 2020, 17, 2327-2333. DOI: https://doi.org/10.1007/s13738-020-01927-w
Hadi, L.; Richard, A.J.O.; Gavin, E.R. J. Am. Soc. Mass Spec. 2004, 15(1), 65-76. https://doi.org/10.1016/j.jasms.2003.09.011
Domingo, P.L.; Garc, B.A.; Leal, J.M. Can. J. Chem. 1990, 68, 228-235. DOI: https://doi.org/10.1139/v90-030
Chowdhury, S.; Rakshit, A.; Acharjee, A.; Ghosh, A.; Mahali, K.; Saha, B. Tenside. Surfact. Det. 2020, 57, 298-309. DOI: https://doi.org/10.3139/113.110696
Chowdhury, S.; Rakshit, A.; Acharjee, A.; Ghosh, A.; Mahali, K.; Saha, B. J. Mol. Liq.
, 290, 111247. DOI: https://doi.org/10.1016/j.molliq.2019.111247
Graciani, M.M.; Rodríguez, M.A.; Moyá, M.L. Int. J. Chem. Kinet. 1997, 29, 377-384. DOI: https://doi.org/10.1002/(SICI)1097-4601(1997)29:5%3C377::AID-KIN8%3E3.0.CO;2-R
Harzion, Z.; Navon, G.; Inorg. Chem. 1980, 19, 2236–2239. Doi: https://doi.org/10.1021/ic50210a008
Khan, M.M.T.; Ramachandraiah, G.; Rao, A.P.; Inorg. Chem. 1986, 25, 665–670. DOI: https://doi.org/10.1021/ic00225a015
Cotton, F.A.; Wilkinson, G. Advanced Inorganic Chemistry- A Comprehensive Text. 6th ed. Wiley Interscience, New York, 1996.
Singh, H.S.; Singh, R.K.; Singh, S.M.; Sisodia, A.K. J. Phys. Chem. 1977, 81, 1044-1047. DOI: https://doi.org/10.1021/j100526a004
Hiremath, G.A.; Timmanagoudar, P.L.; Nandibewoor, S.T. React. Kinet. Catal. Letts. 1998, 63, 403-408. DOI: https://doi.org/10.1007/BF02475419
Kamble, D.L.; Nandibewoor, S.T. J. Phys. Org. Chem. 1998, 11, 171-176. DOI: https://doi.org/10.1002/(SICI)1099-1395(199803)11:3<171::AID-POC988>3.0.CO;2-4
Naik, R.M.; Kumar, B. J. Dis. Sci. Technol. 2012, 33, 647-653. DOI: https://doi.org/10.1080/01932691.2011.579823
Bunton, C.A.; Nome, F.; Quina, F.H.; Romsted, L.S. Acc. Chem. Res. 1991, 24, 357-364. Doi: https://doi.org/10.1021/ar00012a001
Brinchi, L.; Profio, P.D.; Germani, R.; Savelli, G.; Tugliani, M.; Bunton, C.A. Langmuir 2000, 16, 10101-10105. DOI: https://doi.org/10.1021/la000799s
Lopez-Cornejo, P.; Mozo, J.D.; Rolda, ́ N.E.; Domínguez, M.; ́ Sanchez, F. Chem. Phys. Lett. 2002, 352, 33-38. DOI: https://doi.org/10.1016/s0009-2614(01)01287-8
Piszkiewicz, D. J. Am. Chem. Soc. 1997, 99, 7695-7697; DOI: https://doi.org/10.1021/ja00465a046
Sen, P.K.; Gani, N.; Pal, B. Ind. Eng. Chem. Res. 2013, 52, 2803-2813. DOI: https://doi.org/10.1021/ie302656d
Jimenez, R.; Bueno, E.; Cano, I.; Corbacho, E. Int. J. Chem. Kinet. 2004, 26, 627-633. DOI: https://doi.org/10.1002/kin.20038
Downloads
Additional Files
Published
Issue
Section
License
Copyright (c) 2023 ABHISHEK SRIVASTAVA, Manjusha, Neetu Srivastava, Radhey Mohan Naik

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









