A Kinetic and Mechanistic Study of Oxidative Degradation of 2-Aminophenol in Aqueous Alkaline Medium and Antimicrobial Study of Degradation Product 2-Aminophenoxazin-3-One
DOI:
https://doi.org/10.29356/jmcs.v66i3.1756Keywords:
2-aminophenol, hexacyanoferrate (III) ions, oxidized product, antibacterial activity, highly efficientAbstract
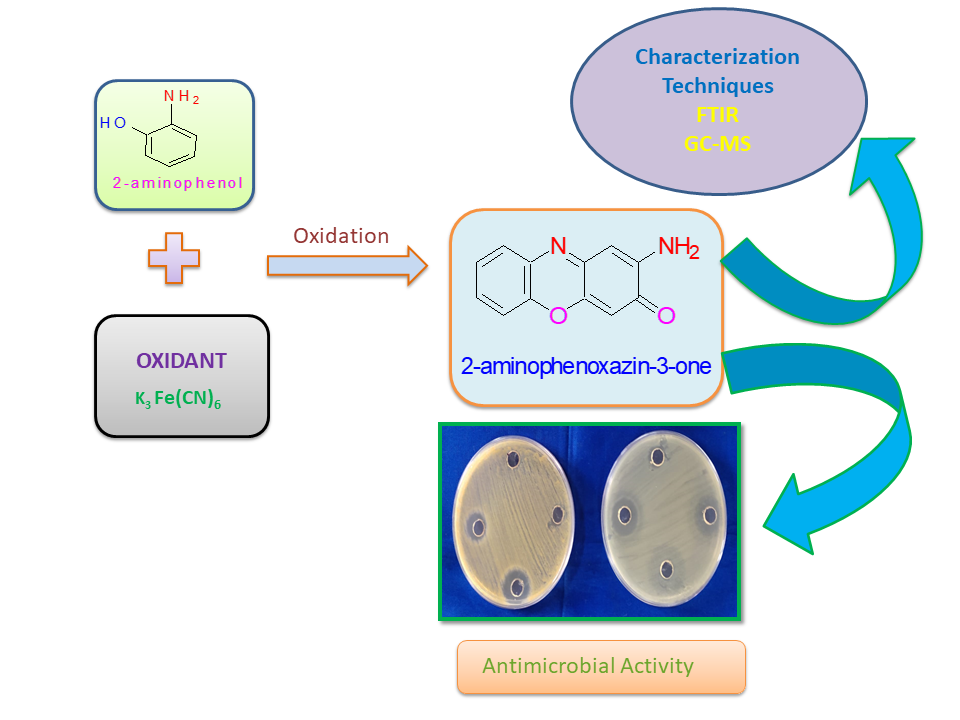
Abstract. The oxidation reaction of a model environmental pollutant, 2-AP to 2-aminophenoxazin-3-one initiated by hexacyanoferrate (III) ion, abbreviated (HCF (III), has been investigated at 25 °C. The 2-aminophenoxazin-3-one (2-AHP) acts as a good antibacterial agent. The experimental data, within the pH range 7.5 to 10 were analyzed. The oxidation of 2-aminophenol was followed kinetic- spectrophotometrically and the initial rates were determined using plane mirror method. The effect of temperature variation on the overall rates was studied at pH = 9 within the range 298-313 K and the corresponding activation energy were evaluated. The rate constant, activation energy, enthalpy, entropy, pre-exponential factor and free energy are: kobs = 11.7 x 10-5 min-1, Ea# = 8.24 kcal/mol, ΔH# = 7.63 kcal/mol, ΔS# = -31.5 e.u, A = 23.7 L mol-1 s-1 and ΔF# = 17.2 kcal /mol. The oxidized product 2-aminophenoxazin-3-one was identified by the FTIR and GC-MS methods of analysis. Antimicrobial activity study of product (2-AHP) with gram positive (S. aureus) and gram-negative bacteria (E. coli) by Agar well diffusion method has been made. The study reveals that antibacterial activity of 2-AHP is more for Staphylococcus aureus (gram positive bacteria) as compared to E. coli (gram negative) bacteria. Thus, the present method is simple, efficient and environmentally friendly for the degradation of 2-aminophenol.
Resumen. Se investigó a reacción de oxidación de un contaminante ambiental modelo, el 2-aminofenol a 2-aminofenoxazin-3-ona (2-AHP) iniciada por el ion hexacianoferrato (III), (HCF (III), a 25 °C, compuesto que actúa como un buen agente antibacteriano. Se analizaron los datos experimentales, dentro del rango de pH de 7,5 a 10. La cinética de la oxidación del 2-aminofenol se siguió espectrofotométricamente y la rapidez inicial se determinó utilizando el método de espejo plano. Se estudió el efecto de la variación de la temperatura sobre rapidez de reacción global a pH = 9, dentro del rango de temperatura de 298 a 313 K y se evaluó la energía de activación correspondiente. La constante de rapidez, la energía de activación, la entalpía, la entropía, el factor preexponencial y la energía libre para esta reacción son: kobs = 11,7 x 10-5 min-1, Ea#= 8,24 kcal/mol, ΔH# = 7,63 kcal/mol, ΔS# = -31,5 e.u, A = 23,7 l/mol/s y ΔF# = 17,2 kcal/mol. El producto oxidado, el 2-AHP, fue identificado por los métodos de análisis FTIR y GC-MS. Su actividad antimicrobiana fue evaluada frente a S. aureus (gram positivo) y bacterias gram negativas (E. coli) mediante el método de difusión en pozos de agar. El estudio revela que la actividad antibacteriana de 2-AHP es mayor para S. aureus en comparación con la bacteria E. coli. Por tanto, el presente método es simple, eficiente y respetuoso con el medio ambiente para la degradación de 2-aminofenol.
Downloads
References
Sajjadi, M.; Nasrollahzadeh, M.;Tahsili, M. R. Sep. Purif. Technol. 2019, 227, 115716.
Elliott, D. W.; Zhang, W. X. Environ. Sci. Technol. 2001, 35, 4922-4926.
Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Environ. Sci. Technol. 2001, 50, 7290-7304.
Li, J.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Chem. Soc. Rev. 2018, 47, 2322-2356.
Zhou, X.; Shi, T.; Zhou, H. Appl. Surf. Sci. 2012, 258, 6204-6211.
Goel, A.; Shikha. Environ. Qual. Manag. 2021, 1-9. DOI:10.1002/tqem.21816
Li, Y. P.; Cao, H. B.; Liu, C. M.; Zhang, Y. J. Hazard. Mater. 2007, 148, 158-163.
Dibene, K.; Yahiaoui, I.; Aitali, S.; Khenniche, L.; Amrane, A.; Aissani-Benissad, F. Environ. Technol. 2021, 42, 905-913.
Etaiw, S. E. D. H.; Werida, A. H. J. Appl. Spectros. 2010, 77, 484-490.
Esplugas, S.; Gimenez, J.; Contreras, S.; Pascual, E.; Rodrı́guez, M. Water Res. 2002, 36, 1034-1042.
Gupta, V. K.; Mohan, D.; Suhas; Singh, K. P. Ind. Eng. Chem. 2006, 45, 1113-1122.
Ghosh, P.; Ghime, D.; Lunia, D. Environ. Prot. Eng. 2017, 43.
He, Z.; Song, S.; Ying, H.; Xu, L.; Chen, J. Ultrason. Sonochem. 2007, 14, 568-574.
Salavagione, H. J.; Arias, J.; Garcés, P.; Morallón, E.; Barbero, C.; Vázquez, J. L. J. Electroanal. Chem. 2004, 565, 375-383.
Nagasawa, H. T.; Gutmann, H. R. J. Biol. Chem. 1959, 234, 1593-1599.
Sharanabasamma, K.; Angadi, M. A.; Tuwar, S. M. Open Catal. J. 2011, 4, 1-8.
Shimpi, R.; Fadat, R.; Janrao, D. M.; Farooqui, M. J. Chem. Pharm. Res. 2014, 6, 1011-1019.
Fukuda, G.; Yoshitake, N.; Khan, Z. A.; Kanazawa, M.; Notoya, Y.; Che, X. F.; Akiyama, S. I.; Tomoda, A.; Chakrabarti, S.; Odawara, M. Biol. Pharm. Bul. 2005, 28, 797-801.
Zorrilla, J. G.; Rial, C.; Cabrera, D.; Molinillo, J. M.; Varela, R. M.; Macías, F. A. Mol. 2021, 26, 3453.
Podder, N.; Mandal, S. New J. Chem. 2020, 44, 12793-12805.
El-Khalafy, S. H.; Hassanein, M. T.; Etaiw, S. E. D. H.; El-Din, A. S. B. Arab. J. Chem. 2017, 10, S2829-S2835.
Puiu, M.; Raducan, A.; Oancea, D. Rev. Roum. Chim. 2007, 52, 1039-1044.
Nandiyanto, A. B. D.; Oktiani, R.; Ragadhita, R. Indones. J. Sci. Technol. 2019, 4.1, 97-118.
Goel A.; Abhilasha. I. J.T.A.S. 2016, 8, 76-81.
Downloads
Published
Issue
Section
License
Copyright (c) 2022 Anjali Goel, Monika Rani

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









