Six-Membered Heterocyclic Boronate Esters. Synthesis and Structural Analysis
DOI:
https://doi.org/10.29356/jmcs.v66i4.1718Keywords:
Arylboronic acids, zwitterionic species, boronate esters, hydrogen bonds, crystallographic analysisAbstract
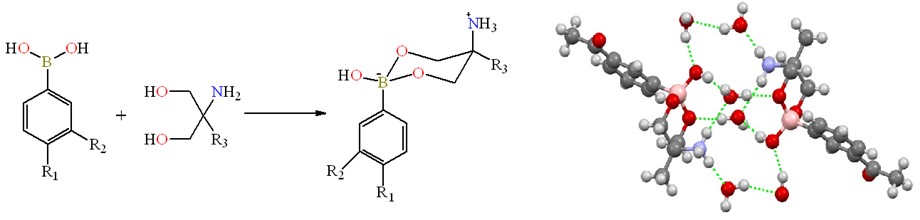
Abstract. Nine heterocyclic zwitterionic boronate esters derived from carbonylphenylboronic acids and amino-diols are described. Compounds were prepared by direct condensation reaction between 3- or 4-formyl/acetylphenylboronic acids with 2-amino-2-methyl-1,3-propanediol (1a-1d) or serinol (2-amino-1,3-propanediol) (1e-1h). Compound 2e was obtained by reaction between 4-formylphenylboronic acid and serinol using a solvent mixture methanol/acetone, an aldol condensation reaction was observed. All compounds were characterized by common spectroscopic techniques such as FT-IR, mass spectrometry, and multinuclear 1H, 13C and 11B NMR spectroscopy. 11B NMR spectra showed signals between δ = 1.9 to 7.3 ppm for all compounds, indicating a tetracoordinated environment for the boron atoms in solution. X-ray diffraction analysis showed that boronates are contained in six-membered heterocycles, which have a chair conformation with -OH and -NH3+ substituents in syn disposition. The formation of channels in the crystal lattice that are filled with water and supported by hydrogen bonding interactions is noteworthy.
Resumen. En el presente trabajo se describen nueve ésteres de boro zwitteriónicos, derivados de ácidos cabonilfenilborónicos. Los compuestos fueron obtenidos mediante reacciones de condensación entre el ácido 3- o 4- formil/acetilfenilborónico con 2-amino-2-metil propanodiol (1a-1d) o serinol (1e-1h). El compuesto 2e se sintetizó a través del ácido 4-formilfenilborónico y serinol (2-amino-1,3-propanodiol) utilizando una mezcla de disolventes metanol/acetona, dando lugar a una reacción de condensación aldólica. Los compuestos fueron caracterizados por técnicas espectroscópicas como son FT-IR, espectrometría de masas y espectroscopia multinuclear de RMN 1H, 13C y 11B. El espectro RMN de 11B mostró señales anchas entre δ = 1.9 y 7.3 ppm para todos los compuestos, lo cual indica la presencia de átomos de boro tetracoordinados en solución. El análisis por difracción de rayos-X de monocristal mostró la formación de heterociclos de 6 miembros en conformación silla, con una marcada estereoselectividad en donde los grupos -OH y -NH3+ se encuentran en disposición syn. En la red cristalina, se observaron canales ocupados por moléculas de agua y soportados por enlaces de hidrógeno.
Downloads
References
Severin, K. Dalton Trans. 2009, 5254-5264. DOI: https://doi.org/10.1039/b902849h
Höpfl, H. Struct. Bonding. 2002, 103, 1-56. DOI: https://doi.org/10.1007/3-540-47808-6_1
Wang, W.; Gao, X.; Wang, B. Curr. Org. Chem. 2002, 6, 14, 1285-1317. DOI: https://doi.org/10.2174/1385272023373446
Springsteen, G.; Wang, B. Tetrahedron. 2002, 58, 26, 5291-5300. DOI: https://doi.org/10.1016/S0040-4020(02)00489-1
Marinez-Aguirre, M. A.; Villamil-Ramos, R.; Guerrero-Alvarez, J. A.; Yatsimirsky A. K. J. Org. Chem. 2013, 78, 10, 4674-4684. DOI: https://doi.org/10.1021/jo400617j
Hall, D. G. Boronic Acids: Preparation and Applications in Organic Synthesis Medicine and Materials, 2nd ed, Wiley-VCH, Weinheim, 2011. DOI: https://doi.org/10.1002/9783527639328
James, T. D; Philips, M. D; Shinkai, S. Boronic acids in Saccaride Recognition Recognition, The Royal Society of Chemistry, 2006. DOI: https://doi.org/10.1039/9781847557612
James, T.D., Shinkai, S. Artificial Receptors as Chemosensors for Carbohydrates. In: Penadés, S. (eds) Host-Guest Chemistry. Topics in Current Chemistry, Vol. 218, Ed., Springer, Berlin, Heidelberg, 2002. DOI: https://doi.org/10.1007/3-540-45010-6_6
James, T. D; Sandanayake, K. R. A; Shinkai, S. Angew. Chem. Int. Ed. Engl. 1996, 35, 1910-1922. DOI: https://doi.org/10.1002/anie.199619101
Jin, S; Cheng, Y; Reid, S; Li, M; Wang, B. Med. Res. Rev. 2010, 30, 171-257. DOI: https://doi.org/10.1002/med.20155
Rowan, S. J; Cantrill, S. J; Cousins, G. R; Sanders, J. K; Stoddart, J. F. Angew. Chem. Int. Ed. 2002, 41, 898-952. DOI: https://doi.org/10.1002/1521-3773(20020315)41:6<898::AID-ANIE898>3.0.CO;2-E
Corbett, P.T.; Leclaire, J.; Vial, L.; West, K.R.; Wietor, J.L.; Sanders, J. K. M.; Otto, S. Chem. Rev. 2006, 106, 3652-3711. DOI: https://doi.org/10.1021/cr020452p
Wilson, A; Gasparini, G; Matile, S. Chem. Soc. Rev. 2014, 43, 1948-1962. DOI: https://doi.org/10.1039/C3CS60342C
Cambre, J, N; Sumerlin, B. S. Polymer. 2011, 52, 4631-4643. DOI: https://doi.org/10.1016/j.polymer.2011.07.057
Dai, C; Cazares, L. H; Wang, L; Chu, Y; Wang, S. L; Troyer, D. A; Semmes, O. J; Drake, R; Wang, B. Chem. Commun. 2011, 47, 10338-10340. DOI: https://doi.org/10.1039/c1cc11814e
Kelly, A. M; Pérez-Fuertes, Y; Arimori, S; Bull, S. D; James, T. D. Org. Lett. 2006, 8, 1971-1974. DOI: https://doi.org/10.1021/ol0602351
Pérez-Fuertes, Y; Kelly, A. M; Johnson, A. L; Arimori, S; Bull, S. D; James, T. D. Org. Lett. 2006, 8, 609-612. DOI: https://doi.org/10.1021/ol052776g
Fujita, N; Shinkai, S; James, T. D. Chem. Asian J. 2008, 3, 1076-1091. DOI: https://doi.org/10.1002/asia.200800069
Nishiyabu, T; Kubo, Y; James, T. D; Fossey, J. S. Chem. Commun. 2011, 47, 1124-1150. DOI: https://doi.org/10.1039/C0CC02921A
Sun, X; Zhai, W; Fossey, J. S; James, T. D. Chem. Commun. 2016, 52, 3456-3469. DOI: https://doi.org/10.1039/C5CC08633G
Bull, S. D; Davidson, M. G; van den Elsen, J. M. H; Fossey, J. S; Jenkins, A. T. A; Jiang, Y.-B; Kubo, Y; Marken, F; Sakurai, K; Zhao, J; James, T, D. Acc. Chem. Res. 2013, 46, 312-326. DOI: https://doi.org/10.1021/ar300130w
Carboni, B; Monnier, L. Tetrahedron. 1999, 55, 1197-1248. DOI: https://doi.org/10.1016/S0040-4020(98)01103-X
Vargas-Díaz, G; Höpfl, H. J. Organomet. Chem. 2009, 694, 3660-3666. DOI: https://doi.org/10.1016/j.jorganchem.2009.07.004
Farfán, N; Höpfl, H; Barba, V; Ochoa, M. E; Santillan, R; Gómez, E; Gutiérrez, A. J. Organomet. Chem. 1999, 581, 70-81. DOI: https://doi.org/10.1016/S0022-328X(99)00054-6
Höpfl, H; Sánchez, M; Farfán, N; Barba, V. Can. J. Chem.1998, 76, 1352-1360. DOI: https://doi.org/10.1139/v98-181
Kubo, Y; Nishiyabu, R; James, T.D. Chem. Commun. 2015, 51, 2005-2020 DOI: https://doi.org/10.1039/C4CC07712A
Salazar-Mendoza, D; Cruz-Huerta, J;Höpfl, H; Ahuactzi, I. F; Sánchez, M. Cryst. Growth Des.2013, 13, 2441-2454. DOI: https://doi.org/10.1021/cg400144t
Sheepwash, E; Zhou, K; Scopelliti, R; Severin, K. Eur. J. Inorg. Chem. 2013, 2013, 2558-2563. DOI: https://doi.org/10.1002/ejic.201300084
Barba, V; Betanzos, I. J. Organomet. Chem. 2007, 692, 4903-4908. DOI: https://doi.org/10.1016/j.jorganchem.2007.07.035
Barba, V; Villamil, R; Luna, R; Godoy-Alcántar, C; Höpfl, H; Beltran, H. I; Zamudio-Rivera, L. S; Santillan, R; Farfán, N. Inorg. Chem. 2006, 45, 2553-2561. DOI: https://doi.org/10.1021/ic051850o
Sheepwash, E; Luisier, N; Krause, M. R; Noé, S; Kubik, S; Severin, K. Chem. Commun. 2012, 48, 7808-7810. DOI: https://doi.org/10.1039/c2cc34231f
Sheepawash, E; Kraml, V; Scopelliti, R; Sereda, O; Neels, A; Severin, K. Angew. Chem. Int. Ed. 2011, 50, 3034 –3037. DOI: https://doi.org/10.1002/anie.201007225
Christinat, N; Scopelliti, R; Severin, K. Angew. Chem. Int. Ed. 2008, 47, 1848-1852. DOI: https://doi.org/10.1002/anie.200705272
Nishimura, N; Yoza, K; Kobayashi, K. J. Am. Chem. Soc. 2010, 132, 777-790. DOI: https://doi.org/10.1021/ja9084918
Barba, V; Höpfl, H; Farfán, N; Santillan, R; Beltran, H. I; Zamudio-Rivera, L. S. Chem. Commun. 2004, 2834–2835. DOI: https://doi.org/10.1039/B410148K
Barba, V; Ramos, P; Jiménez, D; Rivera, A; Meneses, A. Inorg. Chim. Acta. 2013, 401, 30–37. DOI: https://doi.org/10.1016/j.ica.2013.02.033
Celis, N. A; Godoy-Alcántar, C; Guerrero-Álvarez, J; Barba, V. Eur. J. Inorg. Chem. 2014. 1477-1484. DOI: https://doi.org/10.1002/ejic.201301450
Gómez-Jaimes, G; Barba, V. J. Mol. Struct. 2014, 1075, 594-598. DOI: https://doi.org/10.1016/j.molstruc.2014.06.078
González-Hernández, A; Serrano-Melgar, G; Villamil-Ramos, R; Barba, V. Heteroat. Chem. 2017, 28, e21377. DOI: https://doi.org/10.1002/hc.21377
Herrera-España, A.D.; Campillo-Alvarado, G.; Román-Bravo, P.; Herrera-Ruiz, D.; Höpfl, H.; Morales-Rojas, H. Cryst. Growth Des. 2015, 15, 4, 1572-1576. DOI: https://doi.org/10.1021/acs.cgd.5b00219
Campillo-Alvarado, G.; Vargas-Olvera, E. C.; Höpfl, H.; Herrera-España, A. D.; Sánchez-Guadarrama, O.; Morales-Rojas, H.; MacGillivray, L. R.; Rodríguez-Molina, B.; Fárfan N.; Cryst. Growth Des. 2018, 18, 5, 2726-2743. DOI: https://doi.org/10.1021/acs.cgd.7b01368
Sanchez-Portillo, P; Arenaza-Corona, A; Hernandez-Ahuactzi, I. F; Barba, V. J. Mol. Struct. 2017, 1134, 435-443. DOI: https://doi.org/10.1016/j.molstruc.2017.01.013
Sanchez-Portillo, P; Barba, V. ChemistrySelect. 2017, 2, 11265-11272. DOI: https://doi.org/10.1002/slct.201702465
Sánchez-Portillo, P; Hernández-Sirio, A; Godoy-Alcantar, C; Lacroix, P. G; Agarwal, V; Santillán, R; Barba, V. Dyes Pigm. 2021, 186, 108991. DOI: https://doi.org/10.1016/j.dyepig.2020.108991
MestReNova, Nº de versión 12.0.0, 2017, Windows. Mexico: Mestrelab Research.
Dolomanov, V; Bourhis, L. J; Gildea, R. J; Howard, J. A. K; Puschmann, H. J. Appl. Cryst. 2009, 42, 339-341. DOI: https://doi.org/10.1107/S0021889808042726
Sheldrick, G. M. Acta. Cryst C. 2015, 71, 3-8. DOI: https://doi.org/10.1107/S2053229614024218
Edgington, P. R; McCabe, P; Macrae, C. F; Pidock, E; Shields, G. P; Taylor, R; Towler, M; Van De Streek, J. J. Appl. Crystallogr. 2006, 39, 453-457. DOI: https://doi.org/10.1107/S002188980600731X
Höpfl, H; Farfán, N. J. Organomet. Chem. 1997, 547, 71-77. DOI: https://doi.org/10.1016/S0022-328X(97)00183-6
Barba, V; Vargas, G; Gómez, E; Farfán, N. Inorg. Chim. Acta. 2000, 311, 133-137. DOI: https://doi.org/10.1016/S0020-1693(00)00282-6
Rivera, J. M; Rincón, S; Farfán, N; Santillan, R. J. Organomet. Chem. 2011, 696, 2420-2428. DOI: https://doi.org/10.1016/j.jorganchem.2011.03.006
Höpfl, H. J. Organomet. Chem. 1999, 581, 129-149. DOI: https://doi.org/10.1016/S0022-328X(99)00053-4
Rodríguez-Cuamatzi, P; Vargas-Díaz, G; Höpfl, H. Angew. Chem. Int. Ed. 2004, 43, 3041-3044. DOI: https://doi.org/10.1002/anie.200453957
Wang, J; Zheng, L.L; Li, C. J; Zheng, Y. Z; Tong, M. L. Cryst. Growth Des. 2006, 6, 357-359. DOI: https://doi.org/10.1021/cg050388f
Wang, Y. T; Tang, G. M; Liu, Z. M; Yi, X. H. Cryst. Growth Des. 2007, 7, 2272-2275. DOI: https://doi.org/10.1021/cg070487o
Natarajan, R; Charmant, J. P. H; Orpen, A. G; Davis, A. P. Angew. Chem. Int. Ed. 2010, 49, 5125-5129. DOI: https://doi.org/10.1002/anie.201002418

Downloads
Additional Files
Published
Issue
Section
License
Copyright (c) 2022 Ariana León-Negrete, Raúl Villamil-Ramos, Paola Sánchez-Portillo, Arturo González-Hernández, Victor Barba

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









