Photochemical Transformations of Chalcone-Vitamin E Hybrids
PHOTOCHEMICAL TRANSFORMATIONS
DOI:
https://doi.org/10.29356/jmcs.v66i1.1670Keywords:
Photoisomerization, substituent effects, solvent effects, aggregation, deoxyanthocyanidinAbstract
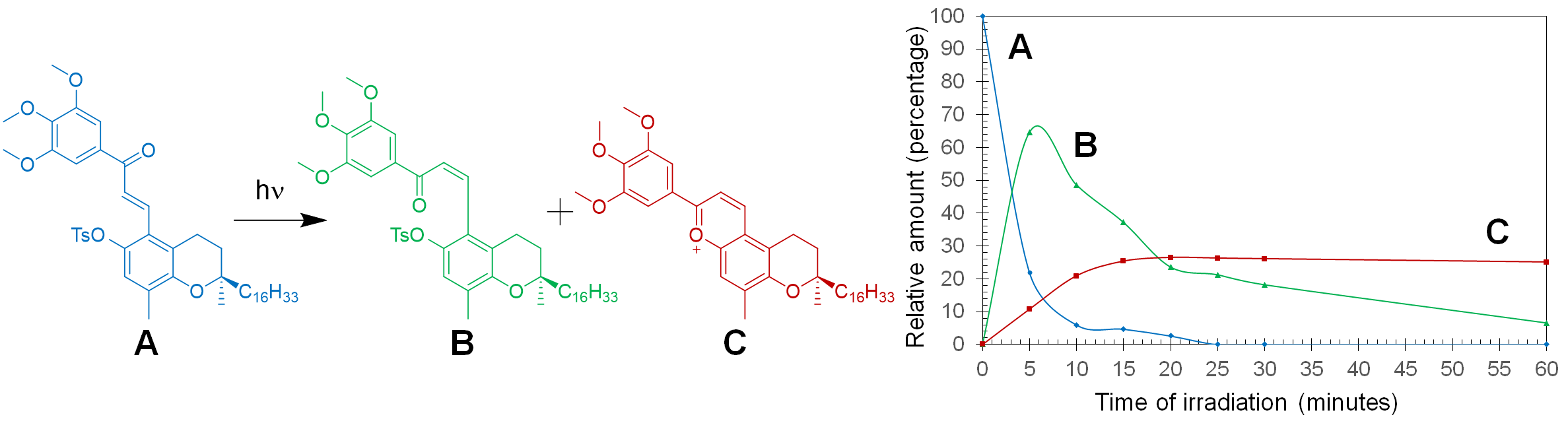
Abstract. Chalcone-vitamin E hybrids 6’-O-tosyl-3,4,5-trimethoxy-δ-tocopherol-chalcone (1), 3,4,5-trimethoxy-δ-tocopherol-chalcone (2), 6’-O-tosyl-3,4,5-trimethoxy-δ-tocopherol-retrochalcone (3) and 3,4,5-trimethoxy-δ-tocopherol-retrochalcone (4) were synthesized as part of a search for new biological activities in these types of derivatives. We report herein on the photoisomerization products of hybrids 1-4, and the effects of the solvent and substitution patterns in producing secondary products such as flavanone 6, 3-deoxyanthocyanidin 8, and hemiketal 10. Photochemically-induced changes are considered important since structural modifications and/or the presence of additional products can affect the biological activity of this type of semisynthetic hybrids.
Resumen. Los híbridos de chalcona-vitamina E, 6’-O-tosil-3,4,5-trimetoxi-δ-tocoferol-chalcona (1), 3,4,5-trimetoxi-δ-tocoferol-chalcona (2), 6’-O-tosil-3,4,5-trimetoxi-δ-tocoferol-retrochalcona (3) y 3,4,5-trimetoxi-δ-tocoferol-retrochalcona (4), fueron sintetizados como parte de la búsqueda de nuevos perfiles de actividad biológica para este tipo de derivados. En este trabajo reportamos los productos de fotoisomerización de los híbridos 1-4, y los efectos del disolvente, así como de distintos patrones de sustitución en la generación de productos secundarios como la flavanona 6, la 3-deoxiantocianidina 8, y el hemicetal 10. Los cambios fotoinducidos son considerados de gran importancia debido a que la modificación en la estructura y/o la presencia de productos adicionales puede afectar la actividad biológica de este tipo de híbridos semisintéticos.
Downloads
References
Wong, W.-Y.; Ward, L. C.; Fong, C. W.; Yap, W. N.; Brown, L. Eur. J. Nutr. 2015, 56, 133–150. DOI: http://dx.doi.org/10.1007/s00394-015-1064-1 DOI: https://doi.org/10.1007/s00394-015-1064-1
Khor, B.-H.; Tiong, H.-C.; Tan, S. C.; Wong, S. K.; Chin, K.-Y.; Karupaiah, T.; Ima-Nirwana, S.; Abdul Gafor, A. H. PLOS ONE 2021, 16, e0255205. DOI: https://doi.org/10.1371/journal.pone.0255205
Jiang, Q. Free Rad. Biol. Med. 2014, 72, 76–90. DOI: https://doi.org/10.1016/j.freeradbiomed.2014.03.035
Harlan, L.; Mena, L. T.; Ramalingam, L.; Jayarathne, S.; Shen, C.-L.; Moustaid-Moussa, N. Nutrients. 2020, 12, 3356. DOI: https://doi.org/10.3390/nu12113356
Reiter, E.; Jiang, Q.; Christen, S. Mol. Aspects Med. 2007, 28, 668–691. DOI: http://dx.doi.org/10.1016/j.mam.2007.01.003 DOI: https://doi.org/10.1016/j.mam.2007.01.003
Shaik, A.; Bhandare, R. R.; Palleapati, K.; Nissankararao, S.; Kancharlapalli, V.; Shaik, S. Molecules 2020, 25, 1047. DOI: http://dx.doi.org/10.3390/molecules25051047 DOI: https://doi.org/10.3390/molecules25051047
Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chem. Rev. 2017, 117, 7762–7810. DOI: http://dx.doi.org/10.1021/acs.chemrev.7b00020 DOI: https://doi.org/10.1021/acs.chemrev.7b00020
Damodar, K.; Kim, J.-K.; Jun, J.-G. Chin. Chem. Lett. 2016, 27, 698–702. DOI: http://dx.doi.org/10.1016/j.cclet.2016.01.043 DOI: https://doi.org/10.1016/j.cclet.2016.01.043
Narender, T.; Papi Reddy, K. Tet. Lett. 2007, 48, 3177–3180. DOI: http://dx.doi.org/10.1016/j.tetlet.2007.03.054 DOI: https://doi.org/10.1016/j.tetlet.2007.03.054
Nicodem, D. E.; de M. G. Matos, J. J. Photochem. 1981, 15, 193–202. DOI: http://dx.doi.org/10.1016/0047-2670(81)87003-7 DOI: https://doi.org/10.1016/0047-2670(81)87003-7
Sidharth, S. N.; Mashitah, M. Y.; Yuvaraj, A. R.; Hui, T. J.; Sarojini, B. K.; Hegde, G. Adv. Mater. Res. 2014, 1033-1034, 1149. DOI: http://dx.doi.org/10.4028/www.scientific.net/AMR.1033-1034.1149 DOI: https://doi.org/10.4028/www.scientific.net/AMR.1033-1034.1149
Maldonado, T.; Ferraudi, G.; Lappin, A. G.; Godoy, F. Chem. PhotoChem. 2018, 2, 95–104. DOI: http://dx.doi.org/10.1002/cptc.201700129 DOI: https://doi.org/10.1002/cptc.201700129
Leydet, Y.; Batat, P.; Jonusauskas, G.; Denisov, S.; Lima, J. C.; Parola, A. J.; McClenaghan, N. D.; Pina, F. J. Phys. Chem. A 2013, 117, 4167–4173. DOI: http://dx.doi.org/10.1021/jp402761j DOI: https://doi.org/10.1021/jp402761j
Pina, F.; Melo, M. J.; Laia, C. A.; Parola, A. J.; Lima, J. C. Chem. Soc. Rev. 2012, 41, 869–908. DOI: http://dx.doi.org/10.1039/C1CS15126F DOI: https://doi.org/10.1039/C1CS15126F
Iwata, S.; Nishino, T.; Inoue, H.; Nagata, N.; Satomi, Y.; Nishino, H.; Shibata, S. Biol. Pharm. Bull. 1997, 20, 1266–1270. DOI: http://dx.doi.org/10.1248/bpb.20.1266 DOI: https://doi.org/10.1248/bpb.20.1266
Yadav, L. D. S. Organic Spectroscopy, Springer, Dordrecht, 2005 (chapter 1), 4. DOI: http://dx.doi.org/10.1007/978-1-4020-2575-4 DOI: https://doi.org/10.1007/978-1-4020-2575-4
Norikane, Y.; Itoh, H.; Arai, T. J. Phys. Chem. A 2002, 106, 2766–2776. DOI: http://dx.doi.org/10.1021/jp0133081 DOI: https://doi.org/10.1021/jp0133081
Matsushima, R.; Kageyama, H. J. Chem. Soc., Perkin Trans. 2. 1985, 6, 743–748. DOI: http://dx.doi.org/10.1039/P29850000743 DOI: https://doi.org/10.1039/P29850000743
Norikane, Y.; Nakayama, N.; Tamaoki, N.; Arai, T.; Nagashima, U. J. Phys. Chem. A. 2003, 107, 8659–8664. DOI: http://dx.doi.org/10.1021/jp027824i DOI: https://doi.org/10.1021/jp027824i
George, F.; Figueiredo, P.; Brouillard, R. Phytochemistry. 1999, 50, 1391–1394. DOI: http://dx.doi.org/10.1016/S0031-9422(98)00427-0 DOI: https://doi.org/10.1016/S0031-9422(98)00427-0
Devia, B.; Llabres, G.; Wouters, J.; Dupont, L.; Escribano-Bailon, M. T.; Pascual-Teresa, S. de; Angenot, L.; Tits, M. Phytochem. Anal. 2002, 13, 114–120. DOI: http://dx.doi.org/10.1002/pca.632 DOI: https://doi.org/10.1002/pca.632
Zhang, X.-J.; Li, L.-Y.; Wang, S.-S.; Que, S.; Yang, W.-Z.; Zhang, F.-Y.; Gong, N.-B.; Cheng, W.; Liang, H.; Ye, M.; Jia, Y.-X.; Zhang, Q.-Y. Tetrahedron. 2013, 69, 11074–11079. DOI: http://dx.doi.org/10.1016/j.tet.2013.11.018 DOI: https://doi.org/10.1016/j.tet.2013.11.018
Pina, F.; Petrov, V.; Laia, C. A. T. Dyes Pigm. 2012, 92, 877–889. DOI: http://dx.doi.org/10.1016/j.dyepig.2011.03.033 DOI: https://doi.org/10.1016/j.dyepig.2011.03.033
Aljan?i?, I. S.; Vu?kovi?, I.; Jadranin, M.; Peši?, M.; ?or?evi?, I.; Podolski-Reni?, A.; Stojkovi?, S.; Menkovi?, N.; Vajs, V. E.; Milosavljevi?, S. M. Phytochemistry. 2014, 98, 190–196. DOI: http://dx.doi.org/10.1016/j.phytochem.2013.11.025 DOI: https://doi.org/10.1016/j.phytochem.2013.11.025
Menezes, J. C. J. M. D. S.; Diederich, M. F. Eur. J. Med. Chem. 2019, 182, 111637. DOI: http://dx.doi.org/10.1016/j.ejmech.2019.111637 DOI: https://doi.org/10.1016/j.ejmech.2019.111637
Kalchevski, D. A.; Petrov, V.; Tadjer, A.; Nenov, A. Phys. Chem. Chem. Phys. 2018, 20, 8924–8934. DOI: http://dx.doi.org/10.1039/C8CP00602D DOI: https://doi.org/10.1039/C8CP00602D
Correa, N. M.; Silber, J. J.; Riter, R. E.; Levinger, N. E. Chem. Rev. 2012, 112, 4569–4602. DOI: http://dx.doi.org/10.1021/cr200254q DOI: https://doi.org/10.1021/cr200254q
Mulinacci, N.; Romani, A.; Pinelli, P.; Gallori, S.; Giaccherini, C.; Vincieri, F. F. Int. J. Pharm. 2001, 216, 23–31. DOI: http://dx.doi.org/10.1016/S0378-5173(00)00685-2 DOI: https://doi.org/10.1016/S0378-5173(00)00685-2
Ionescu, L. G.; De Fávere, V. T., in: Solution Behavior of Surfactants, Mittal, K. L.; Fendler, E. J., Eds., Springer, Boston, 1982, 407-416. DOI: http://dx.doi.org/10.1007/978-1-4613-3491-0_21 DOI: https://doi.org/10.1007/978-1-4613-3491-0_21
Sakhawat, S. S.; Ejaz-ur-Rehman, in: Interactions of Water in Ionic and Nonionic Hydrates, H. Kleeberg, Ed., Springer, Berlin, 1987, 251-255. DOI: http://dx.doi.org/10.1007/978-3-642-72701-6_45 DOI: https://doi.org/10.1007/978-3-642-72701-6_45
Basílio, N.; Pina, F. Molecules 2016, 21, 1502. DOI: http://dx.doi.org/10.3390/molecules21111502 DOI: https://doi.org/10.3390/molecules21111502
Neo, A. G.; López, C.; Romero, V.; Antelo, B.; Delamano, J.; Pérez, A.; Fernández, D.; Almeida, J. F.; Castedo, L.; Tojo, G. J. Org. Chem. 2010, 75, 6764–6770. DOI: http://dx.doi.org/10.1021/jo100742e DOI: https://doi.org/10.1021/jo100742e
Charlton, J. L.; Lai, H. K.; Lypka, G. N. Can. J. Chem. 1980, 58, 458–462. DOI: http://dx.doi.org/10.1139/v80-073 DOI: https://doi.org/10.1139/v80-073

Downloads
Additional Files
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









