Utilization of Biomass (Eucalyptus Lanceolata) as an Adsorbent - Removal of Methylene Blue from Wastewater
DOI:
https://doi.org/10.29356/jmcs.v66i4.1667Keywords:
Activated carbon, adsorption, adsorbent, FTIR, adsorbateAbstract
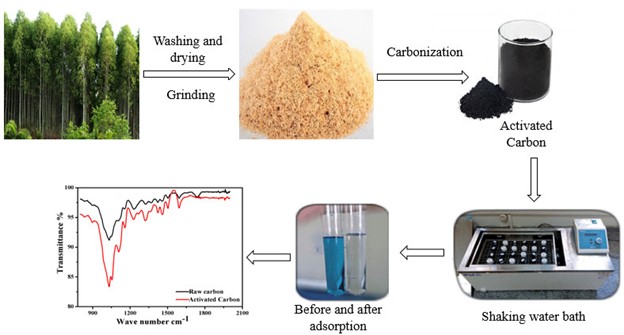
Abstract. Investigation relevant to the influence of activated carbon (AC) supports biomass of Eucalyptus Lanceolata as an adsorbent was not reported previously. The recent work synthesized a series of studies to know various experimental parameters equilibration time and temperature of the adsorbent for the elimination of dye. Physical structure characterization measured that the surface area of activated carbon increased from 399 to 475 m2.g-1 revealed that the raw biomass had more compact surface than those of activated carbon. FTIR bands were found in the region between 3406.29 cm-1 and 3414.0 cm-1 indicates (-OH) stretching group which leads to hydrogen bonding. Adsorption of Methylene blue was studied on raw and activated carbon. The average activation energy of the activated carbon was 33.67 kJ.mol-1, slightly lower than those of raw carbon, which was 49.14 kJ.mol-1. Intraparticle diffusion, Elovich and Bhangam models were applied to evaluate kinetics studies at 20, 30 and 40 ºC. Thermodynamic parameters, ΔH≠, ΔS≠ and ΔE≠ were calculated from the adsorption of kinetics. Results indicate that adsorption is a spontaneous process. The negative value of entropy determined that the acid molecules take an oriented position on the surface of the adsorbent. The results show that all the models were best fitted for adsorption.
Resumen. Una investigación relevante y no reportada previamente respecto a la influencia del carbón activado (CA) apoya que la biomasa de Eucalyptus Lanceolata es un adsorbente. El reciente trabajo sintetizó una serie de estudios para conocer diversos parámetros experimentales de tiempo de equilibrio y temperatura del adsorbente para la eliminación del colorante. La caracterización de la estructura física determinó que el aumento del área superficial del carbón activado de 399 a 475 m2.g-1 revela que la biomasa cruda tenía una superficie más compacta que las del carbón activado. Se encontraron bandas FTIR en la región entre 3406.29 cm-1 y 3414.0 cm-1 indicando el estiramiento del grupo (-OH) que conduce al enlace de hidrógeno. Se estudió la adsorción de azul de metileno en carbón crudo y activado. La energía de activación media del carbón activado fue de 33,67 kJ.mol-1, ligeramente inferior a la del carbón bruto, que fue de 49,14 kJ.mol-1. Se aplicaron modelos de difusión intrapartícula, Elovich y Bhangam para evaluar estudios cinéticos a 20, 30 y 40 ºC. Los parámetros termodinámicos, ΔH≠, ΔS≠ y ΔE≠ se calcularon a partir de la cinética de adsorción. Los resultados indican que la adsorción es un proceso espontáneo. El valor negativo de la entropía determinó que las moléculas de ácido toman una posición orientada en la superficie del adsorbente. Los resultados muestran que todos los modelos se ajustaron mejor para la adsorción.
Downloads
References
Mian, I.; et al. Bioresour. Technol. 2019, 294, 122099. DOI: https://doi.org/10.1016/j.biortech.2019.122099
Bach, Q.-V.; et al. Energy Convers. Manag. 2017, 141, 72-78. DOI: https://doi.org/10.1016/j.enconman.2016.04.097
Kambo, H. S.; Dutta, A. Energy Convers. Manag. 2015, 105, 746-755. DOI: https://doi.org/10.1016/j.enconman.2015.08.031
Rai, H. S.; et al. Crit. Rev. Environ. Sci. Technol. 2005, 35, 219-238. DOI: https://doi.org/10.1080/10643380590917932
Pholosi, A.; Naidoo, E. B.; Ofomaja, A. E. Mater. Chem. Phys. 2019, 222, 20-30. DOI: https://doi.org/10.1016/j.matchemphys.2018.09.067
Caccin, M.; et al. J. Radioanal. Nucl. Chem. 2013. 297, 9-18. DOI: https://doi.org/10.1007/s10967-012-2305-x
Ayranci, E.; Duman, O. J. Hazard. Mater. 2005, 124, 125-132. DOI: https://doi.org/10.1016/j.jhazmat.2005.04.020
Shiau, C. Y.; Pan, C. C. Sep. Sci. Technol. 2005, 39, 1733-1750. DOI: https://doi.org/10.1081/SS-120030779
Dod, R.,; Banerjee, G.; Saini, S. Biotechnol. Bioprocess Eng. 2012, 17, 862-874. DOI: https://doi.org/10.1007/s12257-011-0614-5
Rafatullah, M.; et al. J. Hazard. Mater. 2010, 177, 70-80. DOI: https://doi.org/10.1016/j.jhazmat.2009.12.047
Liu, X.; Wang, Y. Mater. Chem. Phys. 2019, 222, 369-376. DOI: https://doi.org/10.1016/j.matchemphys.2018.10.013
Hameed, B.; Ahmad, A.; Latiff, K. Dyes Pigm. 2007, 75, 143-149. DOI: https://doi.org/10.1016/j.dyepig.2006.05.039
Al-Degs, Y. S.; et al. Dyes Pigm. 2008, 77, 16-23. DOI: https://doi.org/10.1016/j.dyepig.2007.03.001
Alam, S.; et al. Bull. Chem. Soc. Ethiop. 2017, 31, 411-422. DOI: https://doi.org/10.4314/bcse.v31i3.5
Crini, G. Dyes Pigm. 2008, 77, 415-426. DOI: https://doi.org/10.1016/j.dyepig.2007.07.001
Aksu, Z. Process Biochem. 2005, 40, 997-1026. DOI: https://doi.org/10.1016/j.procbio.2004.04.008
Robinson, T.; et al. Bioresource Technol. 2001, 77, 247-255. DOI: https://doi.org/10.1016/S0960-8524(00)00080-8
Pollard, S.; et al. Sci. Total Environ. 1992, 116, 31-52. DOI: https://doi.org/10.1016/0048-9697(92)90363-W
Garg, V. K.; et al. Dyes Pigm. 2004, 63, 243-250. DOI: https://doi.org/10.1016/j.dyepig.2004.03.005
Chiang, Y.-C.; Chiang, P.-C.; Huang, C.-P. Carbon. 2001, 39, 523-534. DOI: https://doi.org/10.1016/S0008-6223(00)00161-5
Pradhan, B. K.; Sandle, N. Carbon. 1999, 37, 1323-1332. DOI: https://doi.org/10.1016/S0008-6223(98)00328-5
Cooke, N. E.; Fuller, O. M.; Gaikwad, R. P. Fuel. 1986, 65, 1254-1260. DOI: https://doi.org/10.1016/0016-2361(86)90238-3
Qasem, N. A.; El-Shaarawi, M. A. Sol. Energy. 2013, 98, 523-542. DOI: https://doi.org/10.1016/j.solener.2013.10.018
Roosta, M.; et al. Spectrochim. Acta, Part A. 2014, 122, 223-231. DOI: https://doi.org/10.1016/j.saa.2013.10.116
Laidler, K.; Suomen Kemistil. A. 1960, 33, 44; Laidler, K. J. in: Chemical Kinetics, McGraw Hill, 2nd Edit, New York, 1965.
Sharma, M.; Vyas, R. K.; Singh, K. Adsorption. 2013, 19, 161-188. DOI: https://doi.org/10.1007/s10450-012-9436-9
Aharoni, C.; Sideman, S.; Hoffer, E. J. Chem. Technol. Biotechnol. 1979, 29, 404-412. DOI: https://doi.org/10.1002/jctb.503290703
Qadeer, R.; et al. J. Chem. Soc. Pak. 1995, 17, 82-86.
Vadivelan, V.; Kumar, K. V. J. Colloid Interface Sci. 2005, 286, 90-100. DOI: https://doi.org/10.1016/j.jcis.2005.01.007
Iqbal, Y.; Khan, M.; Ihsanullah, N. Int. J. Environ. Stud. 2005. 62, 57. DOI: https://doi.org/10.1080/0020723042000253875
Shahin, M. in: Water resources and hydrometeorology of the Arab region, Vol. 59, Springer Science & Business Media, 2007. DOI: https://doi.org/10.1007/1-4020-5414-9
Youssef, A.; El-Nabarawy, T.; Samra, S. Colloids Surf. A. 2004, 235, 153-163. DOI: https://doi.org/10.1016/j.colsurfa.2003.12.017
Zengnian, S.; Minghua, Y. Chin. J. Chem. Eng. 2010, 18, 372-376.
Anirudhan, T.; Suchithra, P. Chem. Eng. J. 2010, 156, 146-156. DOI: https://doi.org/10.1016/j.cej.2009.10.011

Downloads
Published
Issue
Section
License
Copyright (c) 2022 Inamullah Mian, Sultan Alam, Noor Rehman, Hidayat Ullah

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









