The Natural Products and Pharmacological Biodiversity of Brown Algae from the Genus Dictyopteris
DOI:
https://doi.org/10.29356/jmcs.v66i1.1639Keywords:
Marine, natural products, brown algae, Dictyopteris, PhaeophyceaeAbstract
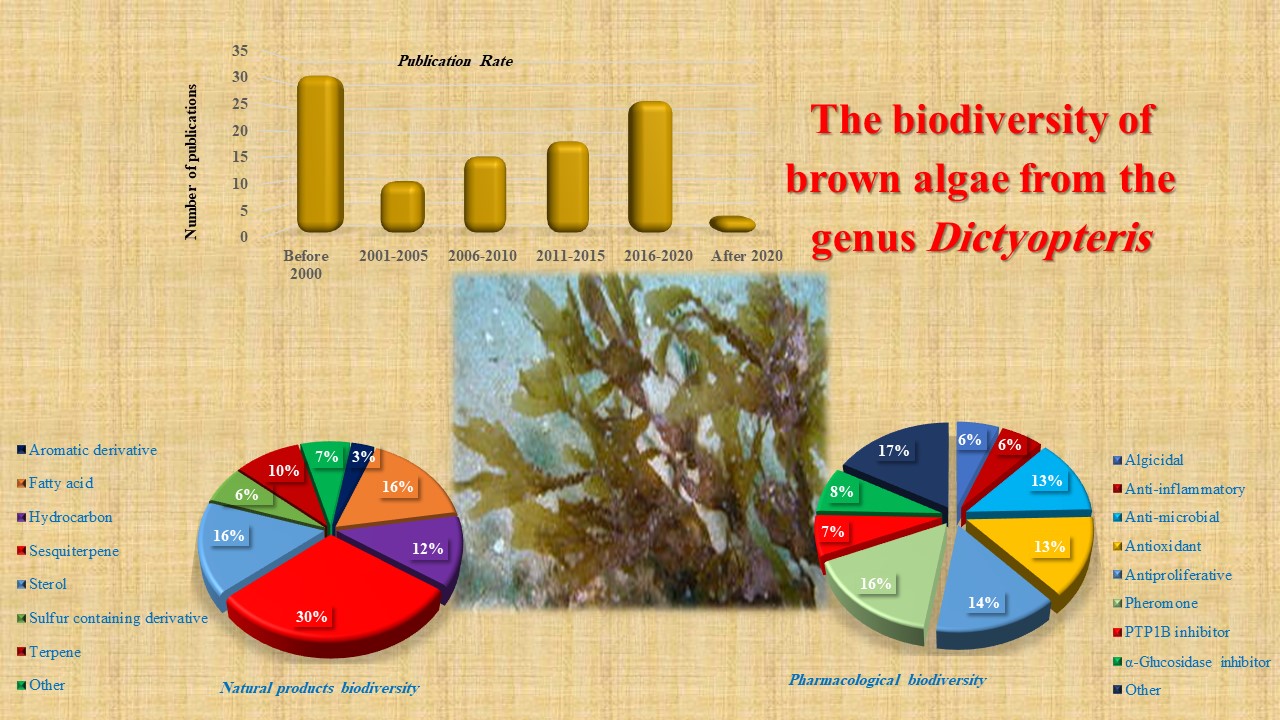
Abstract. Genus Dictyopteris is an important genus among marine seaweeds and is excessively distributed and known by its ocean smell due to its secondary metabolites including C11-hydrocarbons and sulfur compounds. This chemical feature is responsible for its interesting biological properties. This review detected the literature from 1959 to 2021 on the genus Dictyopteris and revealed the secondary metabolites, together with biological activities of the genus Dictyopteris to create the base for additional studies on its clinical and pharmaceutical applications.
Resumen. El género Dictyopteris es un género importante entre las algas marinas y está excesivamente distribuido y conocido por su olor a océano debido a sus metabolitos secundarios que incluyen hidrocarburos C11 y compuestos de azufre. Esta característica química es responsable de sus interesantes propiedades biológicas. Esta revisión detectó la literatura de 1959 a 2021 sobre el género Dictyopteris y reveló los metabolitos secundarios, junto con las actividades biológicas del género Dictyopteris, para crear la base para estudios adicionales sobre sus aplicaciones clínicas y farmacéuticas.
Downloads
References
Rushdi, M. I., et al. Nat. Prod. Res. 2020, 1-19. DOI: 10.1080/14786419.2020.1731741
Nishibayashi, T.; Inoh, S. Bot. Mag. (Tokyo). 1959, 72, 261-268.
Guiry, M.; Guiry, G. AlgaeBase, NUI, Galway. 2021. https://www.algaebase.org/
Moore, R. E. Acc. Chem. Res. 1977, 10, 40-47. DOI: https://doi.org/10.1021/ar50110a002
Boland, W. Proc. Natl. Acad. Sci. U.S.A. 1995, 92, 37-43. DOI: https://doi.org/10.1073/pnas.92.1.37
Wirth, D., et al. Helv. Chim. Acta. 1992, 75, 734-744. DOI: https://doi.org/10.1002/hlca.19920750309
Lever, J., et al. Mar. Drugs. 2020, 18, 142. DOI:10.3390/md18030142
Isroni, W.; Bahri, A. S. IOP Conf. Ser. Earth Environ. Sci. 2020, 493, 012014. DOI: 10.1088/1755-1315/493/1/012014
Moore, R. E.; Pettus, J. A. Tetrahedron Lett. 1968, 4787-4790. DOI: 10.1016/S0040-4039(00)75957-0
Moore, R. E.; Yost, G. J. Chem. Soc. Chem. Commun. 1973, 937-938. DOI: 10.1039/C39730000937
Moore, R. E.; Pettus, J. A.; Mistysyn, J. J. Org. Chem. 1974, 39, 2201-2207. DOI:: 10.1021/jo00929a013
Pickenhagen, W., et al. Helv. Chim. Acta. 1973, 56, 1868-1874. DOI: https://doi.org/10.1002/hlca.19730560607
Moore, R. E. J. Chem. Soc., Chem. Commun. 1971, 1168-1169. DOI: 10.1039/c2971001168b
Schotten, T.; Boland, W.; Jaenicke, L. Tetrahedron Lett. 1986, 27, 2349-2352. DOI: https://doi.org/10.1016/S0040-4039(00)84526-8
Morio, A.; Katsuhiro, K.; Hisashi, T. Bull. Chem. Soc. Jpn. 1994, 67, 1141-1146.
Gosch, B. J., et al. J. Appl. Phycol. 2015, 27, 1607-1622. DOI: 10.1007/s10811-014-0474-4
Vinayak, R. C.; Sabu, A.; Chatterji, A. J. Evid.-Based Integr. Med. 2011, 2011, 673083. DOI: 10.1093/ecam/neq024
Costa, L. S., et al. Biomed. Pharmacother. 2010, 64, 21-28. DOI: https://doi.org/10.1016/j.biopha.2009.03.005
Magalhaes, K. D., et al. Int. J. Mol. Sci. 2011, 12, 3352-3365. DOI: https://doi.org/10.3390/ijms12053352
Rodrigues, S., et al. Biotechnol. Appl. Biochem. 2020, 192, 665–679. DOI: https://doi.org/10.1007/s12010-020-03344-4
König, G. M.; Wright, A. D. Magn. Reson. Chem. 1995, 33, 178-183. DOI: https://doi.org/10.1016/0031-9422(89)80070-6
Hutson, K. S., et al. Int. J. Parasitol. Parasites. 2012, 42, 1135-1141. DOI: https://doi.org/10.1016/j.ijpara.2012.09.007
Sousa, M. B. d., et al. Food Sci. Biotechnol. 2008, 28, 953-958. DOI: https://doi.org/10.1590/S0101-20612008000400030
Khotimchenko, S. V. Phytochemistry. 1995, 38, 1411-1415. DOI: https://doi.org/10.1016/0031-9422(94)00819-F
Cui, Y., et al. Int. J. Biol. Macromol. 2018, 117, 256-263. DOI: 10.1016/j.ijbiomac.2018.05.134
Yang, E.-J., et al. Asian Pac. J. Trop. Biomed. 2014, 4, 529-537. DOI: https://doi.org/10.12980/APJTB.4.2014C1099
Xu, N., et al. J. Appl. Phycol. 2004, 16, 451-456. DOI: 10.1007/s10811-004-5508-x
Xiancui, L., et al. J. Oceanol. Limnol. 2005, 23, 354-356. DOI: 10.1007/BF02847160
Jeon, H., et al. Molecules. 2019, 24, 1420-3049. DOI: 10.3390/molecules24020276
Qiao, Y.-Y., et al. Mar. Drugs. 2009, 7, 600-604. DOI: https://doi.org/10.3390/md7040600
Suzuki, M.; Kowata, N.; Kurosawa, E. Bull. Chem. Soc. Jpn. 1981, 54, 2366-2368, DOI: 10.1246/bcsj.54.2366
Hodgson, D. M.; Salik, S.; Fox, D. J. J. Org. Chem. 2010, 75, 2157-2168. DOI: https://doi.org/10.1021/jo9022974
Kajiwara, T., et al. Phytochemistry. 1989, 28, 636-639. DOI: https://doi.org/10.1016/0031-9422(89)80070-6
McMurry, J. E.; Ko?ovský, P. Tetrahedron Lett. 1985, 26, 2171-2172. DOI: https://doi.org/10.1016/S0040-4039(00)98953-6
Yao, Y.-Q., et al. Cancer Lett. 2008, 264, 127-134. DOI: https://doi.org/10.1016/j.canlet.2008.01.049
Yang, D., et al. Am. J. Cancer Res. 2021, 11, 370-388.
Song, F. H., et al. J. Asian Nat. Prod. Res. 2005, 7, 777-781. DOI: 10.1080/1028602032000169532
Xu, X.-l., et al. Mar. Sci. 2012, 36, 81-84.
Park, S. H., et al. Int. J. Mol. Sci. 2019, 20, 651. DOI: https://doi.org/10.3390/ijms20030651
Yang, H. H., et al. Arch. Pharm. Res. 2015, 38, 876-884. DOI: 10.1007/s12272-014-0435-0
Vizetto-Duarte, C., et al. Phytomedicine. 2016, 23, 550-557. DOI: https://doi.org/10.1016/j.phymed.2016.02.008
Song, F. H., et al. Zhongguo Zhong Yao Za Zhi. 2006, 31, 125-8. DOI: http://www.ncbi.nlm.nih.gov/pubmed/16570798
Lee, J. M., et al. J. Fish. Aquat. Sci. 2020, 23, 1-6.
Hwang, E., et al. Mar. Biotechnol. 2014, 16, 361-370. DOI: 10.1007/s10126-013-9554-8
Rushdi, M. I., et al. RSC Adv. 2020, 10, 24951-24972. DOI: 10.1039/D0RA03576A
Jiang, H., et al. Oncol. Lett. 2018, 15, 3458-3463. DOI: https://doi.org/10.3892/ol.2018.7769
Song, Y., et al. Food Sci. Biotechnol. 2017, 26, 489-494. DOI: 10.1007/s10068-017-0067-5
Kim, K., et al. Int. J. Pharmacol. 2009, 5, 298-306
Rushdi, M. I., et al. S. Afr. J. Bot. 2021, 141, 37-48. DOI: https://doi.org/10.1016/j.sajb.2021.04.018
Cho, J.-Y., et al. Biosci. Biotechnol. Biochem. 1998, 62, 2273-2276. DOI: 10.1271/bbb.62.2273
Peungvicha, P., et al. J. Ethnopharmacol. 1998, 62, 79-84. DOI: https://doi.org/10.1016/S0378-8741(98)00061-0
Wen, W., et al. Molecules. 2009, 14, 2273-7. DOI: http://www.ncbi.nlm.nih.gov/pubmed/19553898
Ji, N. Y., et al. Mar. Drugs. 2009, 7, 355-60. DOI: 10.3390/md7030355
Etsuro, K., et al. Bull. Chem. Soc. Jpn. 1966, 39, 2509-2512. DOI: 10.1246/bcsj.39.2509
Song, F., et al. J. Nat. Prod. 2006, 69, 1261-1266. DOI: 10.1021/np060076u
Song, F., et al. J. Nat. Prod. 2004, 67, 1644-1649. DOI: 10.1021/np040099d
Song, F., et al. J. Nat. Prod. 2005, 68, 1309-1313. DOI: 10.1021/np040227y
Minoru, S., et al. Chem. Lett. 1990, 19, 2187-2190.
Segawa, M.; Yamano, K.; Shirahama, H. Phytochemistry. 1990, 29, 973-974. DOI: https://doi.org/10.1016/0031-9422(90)80058-O
Toshi, I.; Koji, Y.; Tadashi, M. Bull. Chem. Soc. Jpn. 1964, 37, 1053-1055. DOI: 10.1246/bcsj.37.1053
Hay, M. E., et al. Mar. Ecol. . 1988, 48, 185-192.
Schnitzler, I., et al. Oecologia. 2001, 126, 515-521. DOI: https://doi.org/10.1007/s004420000546
Ur Rehman, N., et al. Mar. Drugs. 2019, 17, 666. DOI: https://doi.org/10.3390/md17120666
León-Deniz, L. V., et al. Pharm. Biol. 2009, 47, 864-871.
Ballantine, D. L., et al. Hydrobiologia. 1987, 151, 463-469.
Bianco, É., et al. Molecules. 2013, 18, 5761-5778, DOI: https://doi.org/10.3390/molecules18055761
Syracuse, S. M. Repositório Institucional da UFSC. 2018, https://repositorio.ufsc.br/handle/123456789/191938
Melo, K. R., et al. Molecules. 2013, 18, 14543-63. DOI: 10.3390/molecules181214543
Melo, K. R. T., et al. Holos. 2012, 1, 29-40.
Teixeira, V., et al. Nat. Prod. Commun. 2006, 1, 293- 297.
Rushdi, M. I., et al. S. Afr. J. Bot. 2020, 132, 226-241. DOI: https://doi.org/10.1016/j.sajb.2020.04.031
Yamamoto, Y., et al. Z. Naturforsch. C. 2001, 56, 6-12. DOI: https://doi.org/10.1515/znc-2001-1-202
Kajiwara, T., et al. Z. Naturforsch. C. 2003, 58, 109. DOI: 10.1515/znc-2003-1-219
Yamada, K.; Tan, H.; Hirota, K. Tetrahedron Lett. 1980, 21, 4873-4874. DOI: https://doi.org/10.1016/0040-4039(80)80163-8
Vlachos, V.; Critchley, A.; Von Holy, A. Bot. Mar. 1999, 42, 165-173. DOI: https://doi.org/10.1515/BOT.1999.019
Khallil, A.; Daghman, I.; FadyAA. J. Microbiol. Mod. Tech. 2015, 1, 1- 9
Aoun, Z. B.; Said, R. B.; Farhat, F. Bot. Mar. 2010, 53, 259-264. DOI: https://doi.org/10.1515/BOT.2010.027
Ammar, H. H., et al. Food Chem. 2018, 239, 165-171. DOI: https://doi.org/10.1016/j.foodchem.2017.06.108
Akremi, N., et al. S. Afr. J. Bot. 2017, 108, 308-314. DOI: https://doi.org/10.1016/j.sajb.2016.08.009
Ozdemir, G., et al. Pharm. Biol. 2006, 44, 183-188. DOI: 10.1080/13880200600685949
Dimou, M., et al. J. Nat. Prod. 2016, 79, 584-589. DOI: https://doi.org/10.1021/acs.jnatprod.5b01031
Daskalaki, M. G., et al. Mar. Drugs. 2020, 18, 527. DOI: https://doi.org/10.3390/md18110527
AliAboutabl, E., et al. Med. Aromat. Plant. Sci. Biotechnol. 2010, 4, 41-48
Hofmann, M.; Eichenberger, W. Plant. Cell. .Physiol. 1998, 39, 508-515
Schmid, M., et al. Food Chem. 2018, 265, 70-77. DOI: https://doi.org/10.1016/j.foodchem.2018.05.060
Roller, P.; Au, K.; Moore, R. E. Chem. Commun. 1971, 503-504. DOI: 10.1039/c29710000503
Percival, E.; Anisur Rahman, M.; Weigel, H. Phytochemistry. 1981, 20, 1579-1582. DOI: https://doi.org/10.1016/S0031-9422(00)98535-2
Sokolova, R. V., et al. Chem. Nat. Compd. 2011, 47, 329-334. DOI: 10.1007/s10600-011-9925-1
Kolsi, R. B. A., et al. J. Pharmacogn. Phytochem. 2017, 6, 109-113
Riad, N., et al. Microchem. J. 2020, 152, 104415. DOI: https://doi.org/10.1016/j.microc.2019.104415
Yamada, K., et al. Tetrahedron. 1986, 42, 3775-3780. DOI:10.1016/S0040-4020(01)87531-1
Yamada, K.; Tan, H.; Tatematsu, H. J. Chem. Soc., Chem. Commun. 1979, 13, 572-573. DOI: 10.1039/C39790000572
Jeong, S.-Y., et al. Nat. Prod. Sci. 2012, 18, 130-136.
Kang, K. A., et al. J. Cancer Prev. 2014, 19, 118-124. DOI: 10.15430/JCP.2014.19.2.118
Feng, M.-T., et al. Phytochemistry. 2018, 146, 25-35. DOI: https://doi.org/10.1016/j.phytochem.2017.11.013
Chen, Z., et al. J. Agric. Food Chem. 2014, 62, 6130-6137.
Yang, F., et al. Fitoterapia. 2018, 130, 241-246.
Breuer, O., et al. Eur. J. Biochem. 1993, 215, 705-710. DOI: 10.1111/j.1432-1033.1993.tb18082.x
Millanvoye-Van Brussel, E., et al. Biochem. J. 2004, 380, 533-539. DOI: https://doi.org/10.1042/bj20040069
Ishibashi, F., et al. Biosci. Biotechnol. Biochem. 2013, 77, 1120-1122. DOI: https://doi.org/10.1271/bbb.130018
Shimizu, H., et al. Biochem. Biophys. Res. Commun. 2015, 457, 718-722. DOI: https://doi.org/10.1016/j.bbrc.2015.01.059
Kumagai, M., et al. Molecules. 2018, 23, 1214. DOI: https://doi.org/10.3390/molecules23051214
Kurata, K.; Taniguchi, K.; Suzuki, M. Phytochemistry. 1996, 41, 749-752. DOI: 10.1016/0031-9422(95)00651-6
Dave, M.-N., et al. Heterocycles. 1984, 22, 2301-2307
Wang, H.-S., et al. Eur. J. Org. Chem. 2018, 2018, 915-925. DOI: 10.1002/ejoc.201800026
Laube, T., et al. Tetrahedron. 2002, 58, 4299-4309. DOI: https://doi.org/10.1016/S0040-4020(02)00346-0
Shaikh, H. S. N.a. J. Adv. Res. Rev. 2020, 3, 169-181.
Fenical, W., et al. Phytochemistry. 1972, 11, 1161-1163. DOI: https://doi.org/10.1016/S0031-9422(00)88472-1
Fenical, W., et al. J. Org. Chem. 1973, 38, 2383-2386. DOI: 10.1021/jo00953a022
Yamada, S., et al. PloS one. 2014, 9, e113509. DOI: https://doi.org/10.1371/journal.pone.0113509
Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









