Ab initio study of the mechanism of the reaction ClO + O --> Cl + O2
DOI:
https://doi.org/10.29356/jmcs.v66i1.1628Keywords:
Electronic structure, reaction mechanism, ozone depleting reactionAbstract
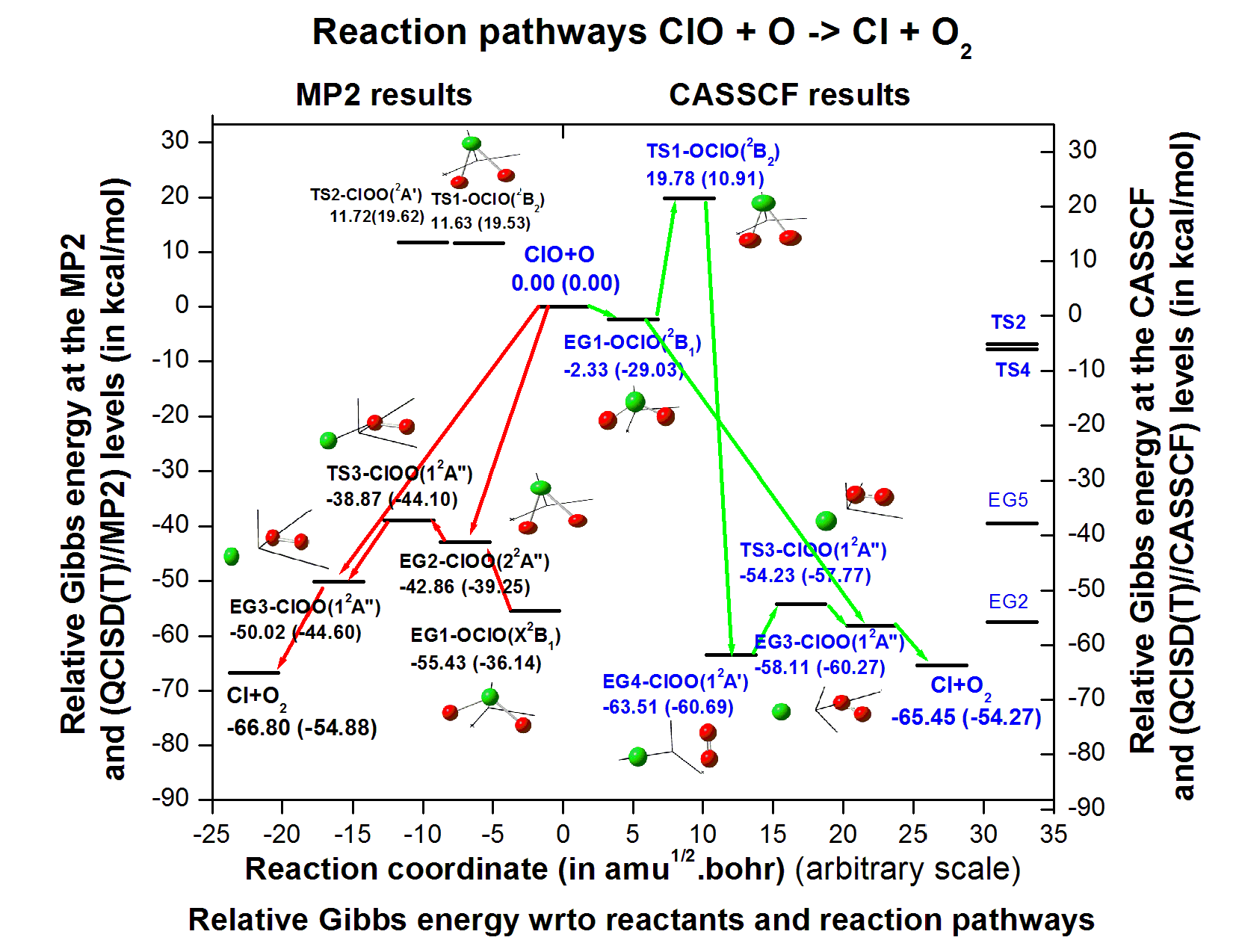
Abstract. Ab initio investigation on the reaction mechanism of ClO + O --> Cl + O2 reaction has been performed using correlation consistent triple zeta basis set. The geometry and frequency of the reactants, products, minimum energy geometries and transition states are obtained using MP2 method and energetics are obtained at the QCISD(T)//MP2 level of theory. Primarily, a possible reaction mechanism is obtained on the basis on IRC calculations using MP2 level of theory. To obtain true picture of the reaction path, we performed IRC calculations using CASSCF method with a minimal basis set 6-31G**. Some new equilibrium geometries and transition states have been identified at the CASSCF level. Energetics are also obtained at the QCISD(T)//CASSCF method. Possible reaction paths have been discussed, which are new in literature. Heat of reaction is found to be consistent with the experimental data. Bond dissociation energies to various dissociation paths are also reported.
Downloads
References
Molina, M. J.; Rowland, F. S. Nature. 1974, 249, 810-812. DOI: https://doi.org/10.1038/249810a0
Stolarski, R. S.; Cicerone, R. J. Can J. Chem. 1974, 52, 1610-1615. DOI: https://dx.doi.org/10.1139/v74-233 DOI: https://doi.org/10.1139/v74-233
Schwab, J. J.; Toohey, D. W.; Brune, W. H.; Anderson, J. G. J. Geophys. Res. 1984, 89, 9581-9587. DOI: https://dx.doi.org/10.1029/JD089iD06p09581 DOI: https://doi.org/10.1029/JD089iD06p09581
Ongstad, A. P.; Birks, J. W. J. Chem. Phys. 1986, 85, 3359-3368. DOI: https://dx.doi.org/10.1063/1.450957 DOI: https://doi.org/10.1063/1.450957
Goldfarb, L.; Burkholder, J. B.; Ravishankara, A. R. J. Phys. Chem. A. 2001, 105, 5402-5409. DOI: https://dx.doi.org/10.1021/jp0100351 DOI: https://doi.org/10.1021/jp0100351
Arkell, A.; Schwager, I. J. Am. Chem. Soc. 1967, 89, 5999-6006. DOI: https://dx.doi.org/10.1021/ja01000a001 DOI: https://doi.org/10.1021/ja01000a001
Gole, J. L. J. Phys. Chem. 1980, 84, 1333-1340. DOI: https://dx.doi.org/10.1021/j100448a009 DOI: https://doi.org/10.1021/j100448a009
Johnsson, K.; Engdahl, A.; and Nelander, B. J. Phys. Chem. 1993, 97, 9603-9606. DOI: https://dx.doi.org/10.1021/j100140a013 DOI: https://doi.org/10.1021/j100140a013
Miyazaki, K.; Tanoura, M.; Tanaka, K.,; Tanaka, T. J. Mol. Spectrosc. 1986, 116, 435-449. DOI: https://dx.doi.org/10.1016/0022-2852(86)90138-4 DOI: https://doi.org/10.1016/0022-2852(86)90138-4
Muller, H. S. P.; Willner, H. J. Phys. Chem. 1993, 97, 10589-10598. DOI: https://dx.doi.org/10.1021/j100143a013. DOI: https://doi.org/10.1021/j100143a013
Francisco, J. S.; Sander, S. P. J. Chem. Phys. 1993, 99, 2897-2901. DOI: https://dx.doi.org/10.1063/1.465197 DOI: https://doi.org/10.1063/1.465197
Beltrán, A.; Andrés, J.; Noury, S.; Silvi, B. J. Phys. Chem. A. 1999, 103, 3078-3088. DOI: https://dx.doi.org/10.1021/jp983999 DOI: https://doi.org/10.1021/jp983999+
Li, Q. -S.; Lu, S.-F.; Xu, W.-G.; Xie, Y.; Schaefer III, H. F. J. Phys. Chem. A 2002, 106, 12324-12330. DOI: https://dx.doi.org/10.1021/jp020362o DOI: https://doi.org/10.1021/jp020362o
Peterson, K. A.; Werner, H. J. J. Chem. Phys. 1992, 96, 8948-8961. DOI: https://dx.doi.org/10.1063/1.462253 DOI: https://doi.org/10.1063/1.462253
Zhu.; R. S.; Lin, M. C. J. Chem. Phys. 2003, 119, 2075-2082. DOI: https://dx.doi.org/10.1063/1.1585027 DOI: https://doi.org/10.1063/1.1585027
Basis set exchange for quantum chemistry: https://www.basissetexchange.org
Chase, M. W. J. Phys. Chem. Ref. Data, Monograph No.9, NIST-JANAF Thermochemical Tables, 4th ed. 1998.
C. E. Moore, Atomic Energy Levels, NSRDS-NBS 35, Vol. 1, 3, Washington, DC, 1971.
Frisch, M. J.; et al., Gaussian 03W, Gaussian, Inc., Wallingbond, CT, 2003.
Huber, K. P.; Herzberg, G. Molecular Spectra and Molecular Structure: Constants of Diatomic Molecules, Vol. IV, Van Nostrand, New York, 1979. DOI: https://doi.org/10.1007/978-1-4757-0961-2
Peterson, K. A.; Shepler, B. C.; Figgen, D.; Stoll, H. J. Phys. Chem. A. 2006, 110, 13877-13883. DOI: https://dx.doi.org/10.1021/jp065887l DOI: https://doi.org/10.1021/jp065887l
Hassanzadeh, P.; Irikura, K. K.; Johnson III, R. D. J. Phys. Chem. A. 1997, 101, 6897-6902. DOI: https://dx.doi.org/10.1021/jp971007e DOI: https://doi.org/10.1021/jp971007e
Grant, D. J.; Garner III, E. B.; Matus, M. H.; Nguyen, M. T.; Peterson, K. A.; Francisco, J. S.; Dixon, D. A. J. Phys. Chem. A 2010, 114, 4254–4265. DOI: https://dx.doi.org/10.1021/jp911320p DOI: https://doi.org/10.1021/jp911320p

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









