Synthesis of N-benzoyl Amino Esters and N-benzoyl Amino Acids and their Antifungal Activity
DOI:
https://doi.org/10.29356/jmcs.v66i1.1584Keywords:
N-benzoyl amino esters;, N-benzoyl amino acids;, antifungal activity;, A. fumigatus;, F. temperatumAbstract
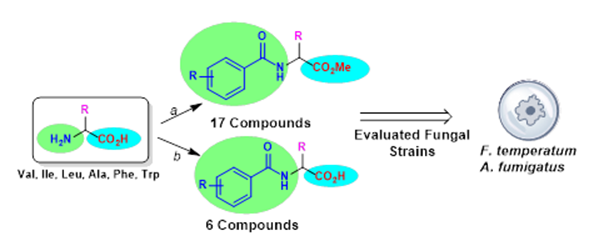
Abstract. A series of N-benzoyl amino esters and N-benzoyl amino acids were synthesized from commercially-available amino acids (Val, Ile, Leu, Ala, Phe, Trp) and were evaluated for their antifungal activity against two filamentous fungi, A. fumigatus and F. temperatum. According to the in vitro assays, five compounds (5-7, 10, 13) exhibited relevant antifungal activity against F. temperatum and two compounds (5 and 7) showed remarkable activity against both fungi strains. Some structure-activity relationships were established regarding the side chain at Ca and the type of substituents on the aromatic ring in the benzoyl moiety. Docking calculations were performed in order to predict binding affinities between compounds prepared herein and fungal chitinase, a potential target against fungi; interactions involving the aromatic rings, the influence on the number of methyl substituents, and configurations on the a-carbon have been analyzed.
Resumen. Una serie de derivados N-benzoilamino ésteres y N-benzoilaminoácidos, sintetizados a partir de aminoácidos disponibles comercialmente (Val, Ile, Leu, Ala, Phe, Trp), se evaluaron como agentes antifúngicos frente a dos hongos filamentosos, A. fumigatus y F. temperatum. De acuerdo con los ensayos in vitro, cinco compuestos (5-7, 10, 13) exhibieron una actividad relevante contra F. temperatum y dos derivados (5 y 7) mostraron una actividad notable contra ambas cepas. Algunas relaciones de estructura actividad permitieron observar el efecto de la cadena lateral del aminoácido, y de los sustituyentes del grupo benzoílo, en la actividad biológica. Se realizaron cálculos de acoplamiento molecular con el propósito de predecir afinidades de enlace entre los compuestos sintetizados y la enzima quitinasa, considerada un blanco molecular potencial. Se analizaron las interacciones que involucran anillos aromáticos, la influencia de los sustituyentes metilo, así como la configuración del Ca.
Downloads
References
Low, C-Y.; Rotstein, C. F1000 Med. Rep. 2011, 3, 1–8 DOI: http://dx.doi.org/10.3410/M3-14 DOI: https://doi.org/10.3410/M3-14
Davies, S. F.; Sarosi, G. A. Postgrad. Med. J. 2016, 73, 242–251. DOI: http://dx.doi.org/10.1080/00325481.1983.11697878 DOI: https://doi.org/10.1080/00325481.1983.11697878
Kainz, K.; Bauer, M. A.; Madeo, F.; Carmona-Gutiérrez, D. MicroB. Cell. 2020, 7, 143–145 DOI: http://dx.doi.org/10.15698/mic2020.06.718 DOI: https://doi.org/10.15698/mic2020.06.718
Fisher, M. C.; Nichola J. H.; N. J.; Sanglard, D.; Gurr, S. H. Science. 2018, 360, 739–742. DOI: http://dx.doi.org/10.1126/science.aap7999 DOI: https://doi.org/10.1126/science.aap7999
Verweij, P. E.; Mellado, E.; Melchers W. J. G. N. Engl. J. Med. 2007, 356, 1481–1483. DOI: http://dx.doi.org/10.1056/NEJMc061720 DOI: https://doi.org/10.1056/NEJMc061720
Walker, L. A.; Gow, N. A. R. Munro, C. A.; Fungal Genet. Biol. 2010, 47, 117–126. DOI: http://dx.doi.org/10.1016/j.fgb.2009.09.003 DOI: https://doi.org/10.1016/j.fgb.2009.09.003
World Health Organization. (?2020)?. First meeting of the WHO antifungal expert group on identifying priority fungal pathogens: meeting report. DOI: https://apps.who.int/iris/handle/10665/332309 (last accessed May, 16th, 2021).
Van Daele, R.; Spriet, I.; Wauters, J.; Maertens, J.; Mercier, T.; Van Hecke, S.; Brüggemann, R.; Med. Mycol. 2019, 57, 328–343. DOI: http://dx.doi.org/10.1093/mmy/myz012 DOI: https://doi.org/10.1093/mmy/myz012
Krebs, H. A.; Wiggins, D.; Stubbs, M.; Sols, A.; Bedoya, F. Biochem. J. 1983, 214, 657–63. DOI: http://dx.doi.org/10.1042/bj214065 DOI: https://doi.org/10.1042/bj2140657
Krátký, M.; Vinšová, J. Molecules. 2012, 17, 9426–9442. DOI: https://doi.org/10.3390/molecules17089426
Berne, S.; Kova?i?, L.; Sova, M.; Kraševec, N.; Gobec, S.; Križaj, I.; Komel, R. Bioorg. Med. Chem. 2015, 23, 4264–4276. DOI: http://dx.doi.org/10.1016/j.bmc.2015.06.042 DOI: https://doi.org/10.1016/j.bmc.2015.06.042
Lima, T. C.; Ferreira, A. R.; Silva, D. F.; Lima E. O.; de Sousa D. P. Nat. Prod. Res. 2018, 32, 572–575. DOI: http://dx.doi.org/doi:10.1080/14786419.2017.1317776 DOI: https://doi.org/10.1080/14786419.2017.1317776
Kaminski, H. M.; Feix, J. B.; Polymers. 2011, 3, 2088–2106. DOI: http://dx.doi.org/10.3390/polym3042088 DOI: https://doi.org/10.3390/polym3042088
Shivakumara, K. N.; Prakasha, K. C.; Gowda, D. C. E-J. Chem. 2009, 6, S473–S479. DOI: http://dx.doi.org/10.1155/2009/267296 DOI: https://doi.org/10.1155/2009/267296
Garg, A.; Borah, D.; Trivedi, P.; Gogoi, D.; Chaliha, A. K.; Ali, A. A.; Chetia, D.; Chaturvedi, V. Sarma, D. ACS Omega. 2020, 5, 29830–29837. DOI: https://doi.org/10.1021/acsomega.0c03862
Sardina, F. J.; Rapoport, H. Chem. Rev. 1996, 96, 1825–1872. DOI: https://doi.org/10.1021/cr9300348
Philip, A.; Kepler, J. A.; Johnson, B. H.; Carroll. F. I. J. Med. Chem. 1988, 31, 870–874. DOI: https://doi.org/10.1021/jm00399a032
Oliva, F.; Bucci, R.; Tamborini, L.; Pieraccini, S.; Pinto, A.; Pellegrino, S. Front. Chem. 2019, 18, 1–10. DOI: http://dx.doi.org/10.3389/fchem.2019.00133 DOI: https://doi.org/10.3389/fchem.2019.00133
Battista, N.; Bari, M.; Bisogno, T.; Biomolecules. 2019, 9, 822. DOI: http://dx.doi.org/10.3390/biom9120822 DOI: https://doi.org/10.3390/biom9120822
Duarte R. C.; Ongaratto, R.; Piovesan, L. A.; de Lima, V. R.; Soldi, V.; Merlo, A. A.; D´Oca, M. G. M. Tetrahedron Lett. 2012, 53, 2454–2460. DOI: https://doi.org/10.1016/j.tetlet.2012.03.015
Abdel-Rahman, A. A. H. Monatsh. Chem. 2008, 139, 289–297. DOI: http://dx.doi.org/10.1007/s00706-007-0770-7 DOI: https://doi.org/10.1007/s00706-007-0770-7
Krátký, M.; Vinšová, J.; Buchta, V.; Horvati, K.; Bösze, S.; Stola?íková, J. Eur. J. Med. Chem. 2010, 45, 6106–6113. DOI: http://dx.doi.org/10.1016/j.ejmech.2010.09.040 DOI: https://doi.org/10.1016/j.ejmech.2010.09.040
Abdel-Wahab, S. M.; Abdelsamii, Z. K.; Abdel-Fattah, H. A.; El-Etrawy, A. S.; Georghiou, P. E. Int. J. Pharm. Chem. 2016, 6, 149–159. DOI: https://doi.org/10.7439/ijpc.v6i6.3292
Prasher, P. Chem. Sci. J. 2016, 7, 847–858. DOI: http://dx.doi.org/10.4172/2150-3494.1000139 DOI: https://doi.org/10.4172/2150-3494.1000139
a) Yoo, W-J.; Li, C-J. J. Am. Chem. Soc. 2006, 128, 13064–13065. DOI: https://doi.org/10.1021/ja064315 b) Tatar, E.; küçükgüzel, I.; Daelemans, D.; Talele, T. T.; Kaushik-Basu, N.; De Clercq, E. Pannecouque, C. Arch. Pharm. 2013, 346, 140–153. DOI: https://doi.org/10.1002/ardp.201200311 c) Trevitt, C. R.; Craven, C. J.; Milanesi, L.; Syson, K.; Mattinen, M-L.; Perkins, J.; Annila, A.; Hunter, C. A.; Waltho J. P. Chem. Biol. 2005, 12, 89–97. DOI: https://doi.org/10.1016/j.chembiol.2004.11.007 d) Anderson, Z, J.; Hobson, C.; Needley, R.; Song, L.; Perryman, M. S.; Kerby, P.; Fox, D. J. Org. Biomol. Chem. 2017, 15, 9372–9378. DOI: https://doi.org/10.1039/C7OB01995E e) Duddeck, H. Magn. Reson. Chem. 2009, 47, 222–227 DOI: https://doi.org/10.1002/mrc.2374
National Committee for Clinical Laboratory Standards 2002. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Conidium-forming Filamentous Fungi. Proposed Standard Document M38-A. Clinical and Laboratory Standards Institute. Wayne, Pa. https://clsi.org/media/1455/m38a2_sample.pdf (last accessed May, 16th, 2021).
SIB. SwissADME. Available online: http://www.swissadme.ch (last accessed April, 23th, 2021).
Daina, A.; Michielin, O.; Zoete, V. Sci. Rep. 2017, 7, 42717. DOI: https://doi.org/4271710.1038/srep42717 DOI: https://doi.org/10.1038/srep42717
Trott, O.; Olson, A. J. J. Comput. Chem. 2010, 31, 455-461. DOI: 10.1002/jcc.21334. DOI: https://doi.org/10.1002/jcc.21334
Li, J.; Sha, Y. Molecules 2008, 13, 1111–1119. DOI: https://doi.org/10.3390/molecules13051111.
Pérez-Picaso, L.; Escalante, J.; Olivo, H. F.; Rios, M. Y. Molecules. 2009, 14, 2836–2849 DOI: https://doi.org/10.3390/molecules14082836
Martynenko, Y. V.; Kazunin, M. S.; Nosulenko, I. S.; Berest, G. G.; Kovalenko, S. I. Kamyshnyi, O. M.; Polishchuk, N. M. Zaporož. med. ž. 2018, 20, 413–420. DOI: https://doi.org/10.14739/2310-1210.2018.3.130544
Robles-Barrios K. F.; Medina-Canales M. G.; Rodríguez-Tovar A. V.; Octavio-Pérez N. Mycopathologia. 2007, 163, 31–39. DOI: https://doi.org/10.1007/s11046-006-0082-1
Latgé, J-P. Clin. Microbiol. Rev. 1999, 12, 310–350. DOI: https://doi.org/10.1128/CMR.00140-18 DOI: https://doi.org/10.1128/CMR.12.2.310
Li, Y.; Sun, H.; Zhu, X.; Bian, C.; Wang, Y.; Si, S. Molecules. 2019, 24, 3155; DOI:10.3390/molecules24173155 DOI: https://doi.org/10.3390/molecules24173155

Downloads
Additional Files
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









