Novel and Reusable Mesoporous Silica Supported 4-Methylbenzenesul- fonate-functionalized Ionic Liquids for Room Temperature Highly Efficient Preparation of 2,4,5-Triaryl-1H-imidazoles
DOI:
https://doi.org/10.29356/jmcs.v65i4.1529Keywords:
Mesoporous silica, supported ionic liquid, high efficient, 2,4,5-triaryl-1H-imidazoles, synergetic and recyclable catalystAbstract
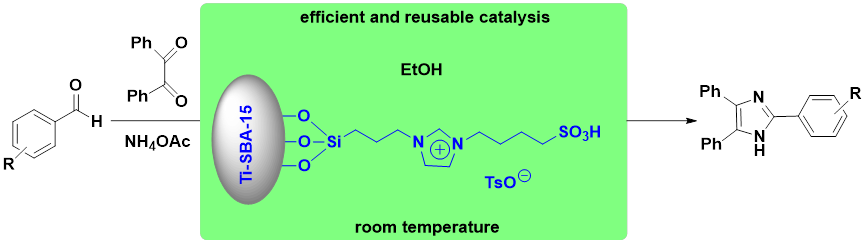
Abstract. A series of mesoporous materials supported ionic liquids were prepared and tested as effective and practical catalysts for the synthesis of 2,4,5-triaryl-1H-imidazoles. The effects of type of catalysts, catalyst amount, and catalyst stability have also been investigated in detail, the catalyst Ti-SBA-15@ILTsO exhibited excellent activity in excellent yields of 92 % ~ 99 % with low catalyst amount at room temperature within 1 h. In addition, the supported ionic liquid can be easily recovered and reused for six times with satisfactory catalytic activity. Furthermore, a general synergetic catalytic mechanism for the reaction was proposed. Maybe this work employing Ti-SBA-15@ILTsO as highly efficient and stable catalyst for the synthesis of 2,4,5-triaryl-1H-imidazoles has potential commercial applications.
Resumen. Se prepararon y probaron una serie de materiales mesoporosos soportados con líquidos iónicos como catalizadores eficaces y prácticos para la síntesis de 2,4,5-triaryl-1H-imidazoles. También se investigaron en detalle los efectos del tipo de catalizadores, la cantidad de catalizador y la estabilidad del catalizador. El catalizador Ti-SBA-15@ILTsO mostró una excelente actividad con rendimientos excelentes del 92 % ~ 99% con una baja cantidad de catalizador a temperatura ambiente en 1 h. Además, el líquido iónico soportado puede recuperarse fácilmente y reutilizarse durante seis veces con una actividad catalítica satisfactoria. Por otro lado, se propuso un mecanismo catalítico sinérgico general para la reacción. Este trabajo que emplea Ti-SBA-15@ILTsO como catalizador altamente eficiente y estable para la síntesis de 2,4,5-triaril-1H-imidazoles puede tener aplicaciones potencialmente comerciales.
Downloads
References
Shabalin, D. A.; Camp, J. E. Org. Biomol. Chem. 2020, 18, 3950-3964. DOI: https://doi.org/10.1039/D0OB00350F
Ren, Z. L.; Cai, S.; Liu, Y. Y.; Xie, Y. Q.; Yuan, D.; Lei, M.; He, P.; Wang, L. J. Org. Chem. 2020, 85, 11014-11024. DOI: https://doi.org/10.1021/acs.joc.0c01454
Wang, L.; Chen, H.; Zhang, N.; Liu, X.; Zheng, K. Tetrahedron Lett. 2021, 64, 152735. DOI: https://doi.org/10.1016/j.tetlet.2020.152735
Jin, X.; Chen, H.; Zhang, W.; Wang, B.; Shen, W.; Lu, H. Appl. Organometal. Chem. 2018, 32, e4577. DOI: https://doi.org/10.1002/aoc.4577
Hojati, S. F.; Nezhadhoseiny, S. A.; Beykzadeh, Z. Monatsh. Chem. 2013, 144, 387-390. DOI: https://doi.org/10.1007/s00706-012-0830-5
Reddy, M. V.; Jeong, Y. T. J. Fluorine Chem.2012, 142, 45-51. DOI: https://doi.org/10.1016/j.jfluchem.2012.06.013
Munsur, A. Z. A.; Roy, H. N.; Imon, M. K. Arab. J. Chem. 2020, 13, 8807-8814. DOI: https://doi.org/10.1016/j.arabjc.2020.10.010
Shaabani, A.; Afshari, R.; Hooshmand, S. E.; Nejad, M. K. ACS Sustain. Chem. Eng. 2017, 5, 9506-9516. DOI: https://doi.org/10.1021/acssuschemeng.7b02741
Jayram, J.; Jeena, V. RSC Adv. 2018, 8, 37557-37563. DOI: https://doi.org/10.1039/C8RA07238H
Kumar, G.; Mogha, N. K.; Kumar, M.; Subodh, Masram, D. T. Dalton Trans. 2020, 49, 1963-1974. DOI: https://doi.org/10.1039/C9DT04416G
Naeimi, H.; Aghaseyedkarimi, D. New J. Chem. 2015, 39, 9415-9421. DOI: https://doi.org/10.1039/C5NJ01273B
Allahresani, A.; Naghdi, E.; Nasseri, M. A. Inorg. Chem. Commun. 2020, 119, 108137. DOI: https://doi.org/10.1016/j.inoche.2020.108137
Shaabani, A.; Afshari, R.; Hooshmand, S. E. New J. Chem. 2017, 41, 8469-8481. DOI: https://doi.org/10.1039/C7NJ01150D
Sangshetti, J. N.; Kokare, N. D.; Kotharkara, S. A.; Shinde, D. B. J. Chem. Sci. 2008, 120, 463-467. DOI: https://doi.org/10.1007/s12039-008-0072-6
Zarnegar, Z.; Safari, J. RSC Adv. 2014, 4, 20932-20939. DOI: https://doi.org/10.1039/C4RA03176H
Waheed, M.; Ahmed, N.; Alsharif, M. A.; Alahmdi, M. I.; Mukhtar, S. ChemistrySelect 2017, 2, 7946-7950. DOI: https://doi.org/10.1002/slct.201701299
Nguyen, T. T.; Le, N. P. T.; Nguyen, T. T.; Tran, P. H. RSC Adv. 2019, 9, 38148-38153. DOI: https://doi.org/10.1039/C9RA08074K
Kumar, D.; Kommi, D. N.; Bollineni, N.; Patel, A. R.; Chakraborti, A. K. Green Chem. 2012, 14, 2038-2049. DOI: https://doi.org/10.1039/c2gc35277j
Vinoth, G.; Indira, S.; Bharathi, M.; Archana, G.; Alves, L. G.; Martins, A. M.; Bharathi, K. S. Inorg. Chim. Acta 2021, 516, 120089. DOI: https://doi.org/10.1016/j.ica.2020.120089
Pervaiz, S.; Mutahir, S.; Ullah, I.; Ashraf, M.; Liu, X.; Tariq, S.; Zhou, B. J.; Khan, M. A. Chem. Biodivers. 2020, 17, e1900493. DOI: https://doi.org/10.1002/cbdv.201900493
Nordness, O.; Brennecke, J. F. Chem. Rev. 2020, 120, 12873-12902. DOI: https://doi.org/10.1021/acs.chemrev.0c00373
Hu, Y.; Zhang, R. L.; Fang, D. Environ. Chem. Lett. 2019, 17, 501-508. DOI: https://doi.org/10.1007/s10311-018-0793-9
Itoh, T.; Takagi, Y. ACS Sustain. Chem. Eng. 2021, 9, 1443-1458. DOI: https://doi.org/10.1021/acssuschemeng.0c07097
Tapia, M. G.; Montes, A. C.; Morcillo, E. M.; Huguet, M. T. G.; de Torres, N. H. W.; Ríos R. C. J. Mex. Chem. Soc. 2014, 58, 16-21.
Doherty, A. P.; Patterson, S.; Diaconu, L.; Graham, L.; Barhdadi, R.; Puchelle, V.; Wagner, K.; Office, D. L.; Chen, J.; Wallace, G. G. J. Mex. Chem. Soc. 2015, 59, 263-268; Guerrero R. L.; Rivero, I. A. J. Mex. Chem. Soc. 2012, 56, 201-206.
Banothu, J.; Gali, R.; Velpula, R.; Bavantula, R. Arab. J. Chem. 2017, 10, S2754-S2761. DOI: https://doi.org/10.1016/j.arabjc.2013.10.022
Hilal, D. A.; Hanoon, H. D. Res. Chem. Intermed. 2020, 46, 1521-1538. DOI: https://doi.org/10.1007/s11164-019-04048-z
Fehrmann, R.; Riisager, A.; Haumann, M. Supported ionic liquids: Fundamentals and applications, Wiley-VCH, Weinheim, 2014; Mohamedali, M.; Ibrahim, H.; Henni, A. Micropor. Mesopor. Mater. 2020, 294, 109916. DOI: https://doi.org/10.1002/9783527654789
Gupta, R.; Yadav, M.; Gaur, R.; Arora, G.; Yadav, P.; Sharma, R. K. Mater. Horiz. 2020, 7, 3097-3130. DOI: https://doi.org/10.1039/D0MH01088J
Sudarsanam, P.; Zhong, R.; den Bosch, S. V.; Coman, S. M.; Parvulescu, V. I.; Sels, B. F. Chem. Soc. Rev. 2018, 47, 8349-8402. DOI: https://doi.org/10.1039/C8CS00410B
Yao, N.; Chen, C.; Li, D. J.; Hu, Y. L. J. Environ. Chem. Eng. 2020, 8, 103953. DOI: https://doi.org/10.1016/j.jece.2020.103953
Kukawka, R.; Pawlowska-Zygarowicz, A.; Dzialkowska, J.; Pietrowski, M.; Maciejew, H.; Bica, K.; Smiglak, M. ACS Sustain. Chem. Eng. 2019, 7, 4699-4706. DOI: https://doi.org/10.1021/acssuschemeng.8b04357
Dhar, A.; Kumar, N. S.; Khimani, M.; Al-Fatesh, A. S.; Ibrahim, A. A.; Fakeeha, A. H.; Patel, H.; Vekariya, R. L. RSC Adv. 2020, 10, 15282-15292. DOI: https://doi.org/10.1039/D0RA00556H
Verma, P.; Kuwahara, Y.; Mori, K.; Raja, R.; Yamashita, H. Nanoscale 2020, 12, 11333-11363; Awoke, Y.; Chebude, Y.; Márquez-Álvarez, C.; Díaz, I. Catal. Today 2020, 345, 190-200; Wang, S.; Li, Z.; Yi, W.; Fu, P.; Zhang, A.; Bai, X. Renew. Energy 2021, 163, 1673-1681; Miao, K.; Luo, X.; Wang, W.; Guo, J.; Guo, S.; Cao, F.; Hu, Y.; Chang, P.; Feng, G. Micropor. Mesopor. Mater. 2019, 289, 109640; Ruchomski, L.; Pikus, S.; Pikula, T.; M?czka, E.; Kosmulski, M. Colloid. Surface. A 2020, 599, 124922.
Dokhaee, Z.; Ghiaci, M.; Farrokhpour, H.; Buntkowsky, G.; Breitzke, H. Ind. Eng. Chem. Res. 2020, 59, 12632-12644. DOI: https://doi.org/10.1021/acs.iecr.0c01050
Huang, Y.; Zheng, K.; Liu, X.; Meng, X.; Astruc, D. Inorg. Chem. Front. 2020, 7, 939-945. DOI: https://doi.org/10.1039/C9QI01449G
Chatterjee, S.; Bhaduri, K.; Modak, A.; Selvaraj, M.; Bal, R.; Chowdhury, B.; Bhaumik, A. Mol. Catal. 2021, 502, 111381. DOI: https://doi.org/10.1016/j.mcat.2020.111381
Ziarani, G. M.; Rohani, S.; Ziarati, A.; Badiei, A. RSC Adv. 2018, 8, 41048-41100. DOI: https://doi.org/10.1039/C8RA09038F
Verma, P.; Kuwahara, Y.; Mori, K.; Raja, R.; Yamashita, H. Nanoscale 2020, 12, 11333-11363. DOI: https://doi.org/10.1039/D0NR00732C

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









