The Inversion Process of 1,3-cyclohexanedione
DOI:
https://doi.org/10.29356/jmcs.v65i3.1521Keywords:
Inversion, topomerization, 1,3-cyclohanedione, conformational analysis, transition stateAbstract
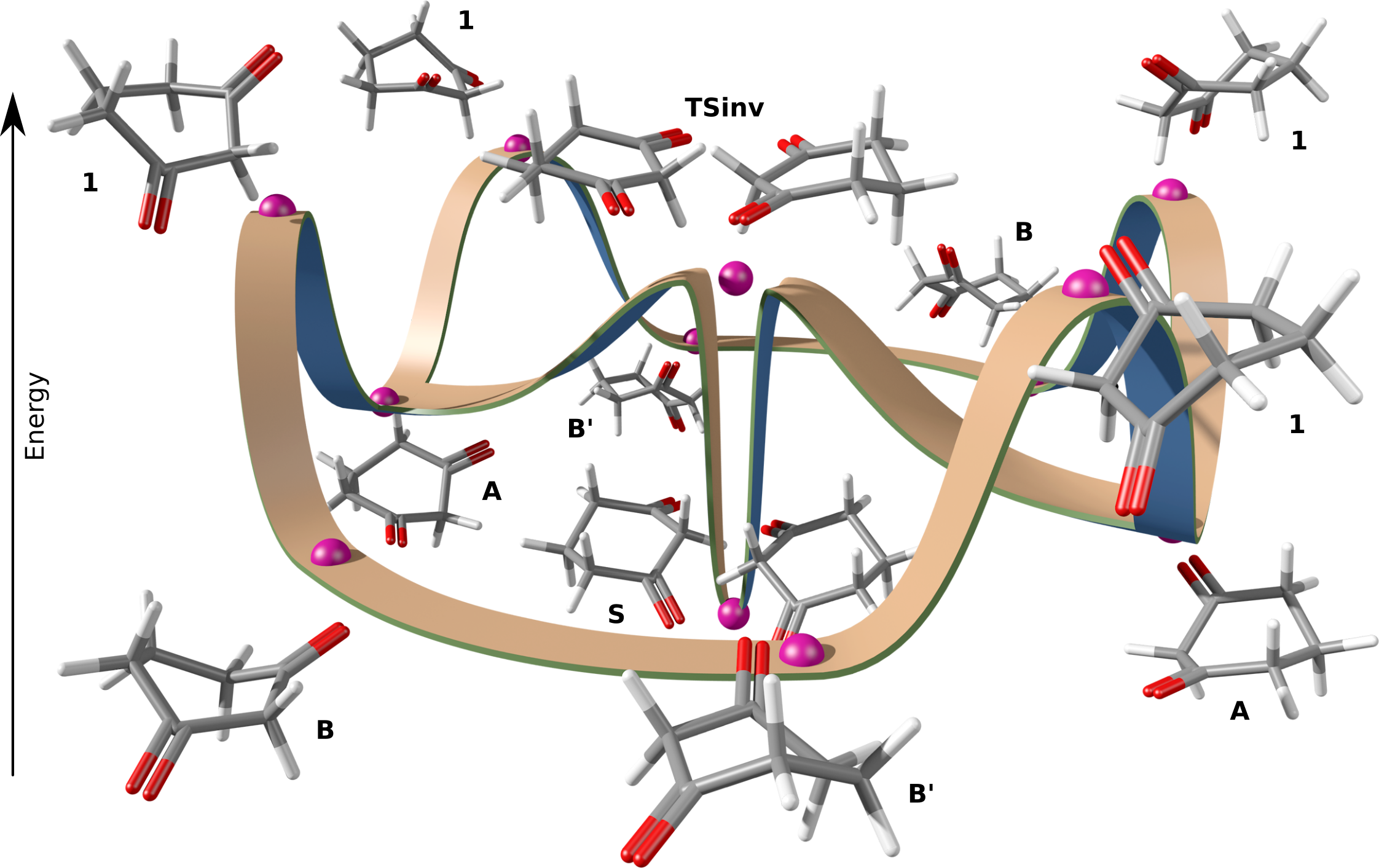
Abstract. The inversion process of 1,3-cyclohexanedione was studied to know the energy associated with the chair-chair interconversion. 1,3-cyclohexanedione has a conformational inversion energy of 1.87 kcal/mol evaluated at M06-2x/6-311++G(2d,2p) level of theory. The global process combines inversion and topomerization originated by boat-boat interconversion that includes only two trajectories to the inversion transition state but no six-like cyclohexane, or four-like oxane and thiane. The process includes two different twisted boats associated with a boat transition state. A global scheme is proposed to represent this conformational equilibrium.
Resumen. Se estudió el proceso de inversión de la 1,3-ciclohexanediona para conocer la energía asociada a la interconversión silla-silla. La 1,3-ciclohexanediona tiene una energía de inversión conformacional de 1.87 kcal/mol evaluada al nivel de teoría M06-2x/6-311++G(2d,2p). El proceso global combina la inversión y la topomerización originada por la interconversion entre dos confórmeros de bote, que incluye sólo dos trayectorias que conectan con el estado de transición de inversión, a diferencia del ciclohexano que tiene seis, y el oxano y el tiano que cuentan con cuatro. El proceso incluye dos estructuras de botes torcido diferentes asociados a un estado de transición de bote. Se propone un esquema global para representar este equilibrio conformacional.
Downloads
References
Melchor-Martínez, E.M.; Silva-Mares, D.A.; Torres-Lopez, E.; Waksman, N.; Guido, P.; Shao-Nong, Ch.; Niemitz, M.; Sanchez-Castellanos, M.; Toscano, R.; Cuevas, G.; Rivas-Galindo, V.M. J. Nat. Prod. 2017, 80, 2252-2262. DOI: doi.org/10.1021/acs.jnatprod.7b00193. DOI: https://doi.org/10.1021/acs.jnatprod.7b00193
Silva-Mares, D.A.; Torres-Lopez, E.; Rivas-Estilla, A.M.; Cordero-Perez, P.; Waksman, N.; Rivas-Galindo, V.R. Nat. Prod. Commun. 2013, 8, 297-298. DOI: doi.org/10.1177/1934578X1300800305. DOI: https://doi.org/10.1177/1934578X1300800305
Fernández-Alonso, M. C.; Asensio, J.L.; Cañada, F.J.; Jiménez-Barbero, J.; Cuevas, G. Chem. Phys. Chem. 2003, 4, 748-753. DOI: doi.org/10.1002/cphc.200200547 DOI: https://doi.org/10.1002/cphc.200200547
Fernández-Alonso, M. C.; Cañada, J.; Jiménez-Barbero, J.; Cuevas, G. Chem. Phys. Chem. 2005, 6, 671-681. DOI: doi.org/10.1002/cphc.200400495. DOI: https://doi.org/10.1002/cphc.200400495
Eliel, E.L.; Wilen, S.H. Stereochemistry of organic compounds, Wiley-Interscience, New York, 1994, 689. ISBN: 978-0-471-01670-0.
Carey, F.A.; Sundberg, R.J. Advanced Organic Chemistry. Part A. Structure and Mechanisms, Plenium Press, New York. 1990, 132. ISBN: 978-0-387-44899-2.
Anslyn, E.V.; Dougherty D.A. Modern Physical Organic Chemistry, University Science Books. Sausalito California 2006, 107. ISBN-13: 978-189138931.
Yogev, A.; Mazur, Y. J. Org. Chem. 1967, 32, 2162-2166. DOI: doi.org/10.1021/jo01282a018. DOI: https://doi.org/10.1021/jo01282a018
Olah, G.A.; Prakash, G.K.S.; Arvanahi, M.; Anet, F.A.L. J. Am. Chem. Soc. 1982, 104, 7105-7108. DOI: doi.org/10.1021/ja00389a037. DOI: https://doi.org/10.1021/ja00389a037
Biarnés, X.; Nieto, J.; Planas, A.; Rovira, C. J. Biol. Chem. 2006, 281, 1432-1441. DOI: doi.org/10.74/jbc.M507643200. DOI: https://doi.org/10.1074/jbc.M507643200
Khodabandeh, M. H.; Rezaeianpour, S.; Davari, M.D.; Sakhaee, N.; Zare, K.; Anary, M.; Naderi, F. J. Theor. Comp. Chem. 2014, 13, 1450047. DOI: doi.org/10.1142/S0219633614500473. DOI: https://doi.org/10.1142/S0219633614500473
Wang, Z. Experimental and kinetic modeling study of cyclohexane and its mono-alkylated derivatives combustion. Springer Thesis. ISBN 978-981-10-5692-5 2018. DOI: https://doi.org/10.1007/978-981-10-5693-2
Bain, A.D.; Baron, M.; Burger, S.K.; Kowalewski, V.J.; Rodríguez M.B. J. Phys. Chem. 2011, 115, 9207-9216. DOI: doi.org/10.1021/jp205375f. DOI: https://doi.org/10.1021/jp205375f
Alamairy, M.A.; Benniston, A.C.; Copley, G.; Harriman, A.; Howgego, D. J. Phys. Chem. 2011, 115, 12111-12119. DOI: doi.org/10.1021/jp2070419. DOI: https://doi.org/10.1021/jp2070419
Chun, H.J.; Ocola, E.J.; Laane, J. J. Phys. Chem. A. 2016, 120, 7677-7680. DOI: doi.org/10.1021/acs.jpca.6b08727. DOI: https://doi.org/10.1021/acs.jpca.6b08727
During, J.R.; Ward, R.M.; Guirgis, G.A.; Gounev. J. Raman- Spectrosc. 2009, 40, 1919-1930. DOI: doi.org/10.1002/jrs.2341. DOI: https://doi.org/10.1002/jrs.2341
Kuo, C.-M.; Bezuidenhoudt, B.C.B.; Conradie, J. J. Phys. Org. Chem. 2013, 26, 327-334. DOI: doi.org/10.1002/poc.3092. DOI: https://doi.org/10.1002/poc.3092
Han, S.; Yoo, H.S.; Ahn, A.-S.; Choi, Y.S.; Kim, S.K. Chem. Phys. Lett. 2011, 518, 38-43. DOI: doi.org/10.1016/j.cplett.2011.11.005. DOI: https://doi.org/10.1016/j.cplett.2011.11.005
Yao, X. X.; Wang, J.B.; Yao, Q.; Li, Y.Q.; Li, Z.R.¸Li, X.Y. Comb Flam. 2019, 204, 176-188. DOI: doi.org/10.1016/j.combustflame.2019.03.01117. DOI: https://doi.org/10.1016/j.combustflame.2019.03.011
Bian, H.; Wang, A.; Sun, J.; Zhang, F. Proc. Comb. Inst. 2016. 1-8. DOI: doi.org/10.1016/j.proci.2016.07.049.
Bian, H.; Ye, L.; Zhong, W. Tetrahedron. 2019, 74, 449-457. DOI: doi.org/10.1016/j.tet.2018.12.020. DOI: https://doi.org/10.1016/j.tet.2018.12.020
Bian, H.; Ye, L.; Li, J.; Sun, J.; Liang, T.; Zhong, W.; Zhao, J. Com. Flam. 2019, 205, 193-205. DOI: doi.org/10.1016/j.combustflame.2019.04.024. DOI: https://doi.org/10.1016/j.combustflame.2019.04.024
Bian, H.; Zhang, Y.; Wang, Y.; Zhao, J.; Ruan, X.; Li, J. Int. J. Quantum Chem. 2021, 26636. DOI: doi.org/10.1002/qua.26636.
Stortz, C.A. J. Phys. Org. Chem. 2010, 23, 1173-1186. DOI: 10.1002/poc.1689. DOI: https://doi.org/10.1002/poc.1689
Barquera-Lozada, J.E.; Cuevas. G. Computational simulation of terminal biogénesis of sesquiterpenes: the case of 8-epiconfertin. In Quantum Biochemistry. Estructure and Biological Activity. Matta Cherif Ed., Wiley-VCH, New York. 2009, 623-650. ISBN: 978-3-527-32322-7. DOI: https://doi.org/10.1002/9783527629213.ch22
Barquera-Lozada, J.E.; Quiroz-Garcia B.; Quijano, L.; Cuevas, G. J. Org. Chem. 2010, 75, 2139-2146. DOI: doi.org/10.1021/jo902170w. DOI: https://doi.org/10.1021/jo902170w
Barquera-Lozada, J.E.; Cuevas, G. J. Org. Chem. 2009, 74, 874-883. DOI: doi.org/10.1021/jo802445n. DOI: https://doi.org/10.1021/jo802445n
Frisch, M.J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M. ; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, O.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2013.
Zhao, Y.; Truhlar, D.G. Acc. Chem. Res. 2008, 41, 157–167. DOI: doi.org/10.1021/ar700111a. DOI: https://doi.org/10.1021/ar700111a
Zhao, Y.; Truhlar, D. G. J. Phys. Chem. A. 2008, 112, 1095-1099. DOI: doi.org/10.1021/jp7109127. DOI: https://doi.org/10.1021/jp7109127
Fukui, K. Acc. Che. Res. 1981, 14, 363-368. DOI: doi.org/10.1021/ar00072a001. DOI: https://doi.org/10.1021/ar00072a001
Oki, M. Application of Dynamic NMR Spectroscopy (Methods in Stereochemistry Analysis), Wiley-VCH, 1985. ISBN-13: 978-0895731203
Bernard, M.; Canuel, L.; St. Jacques, M. J. Am. Chem. Soc. 1974, 96, 2929-2936. DOI: doi.org/10.1021/ja00816a044. DOI: https://doi.org/10.1021/ja00816a044
Kwart, H.; Rock, M.C.; Sanchez-Obregon, R.; Walls, F. J. Am. Chem. Soc. 1972, 94, 1759-1760. DOI: doi.org/10.1021/ja00760a064. DOI: https://doi.org/10.1021/ja00760a064
Anderson, V.E. Ground State Destabilization. In eLS, Ed., Wiley, 2001. DOI: doi.org/10.1038/npg.els.0000625. DOI: https://doi.org/10.1038/npg.els.0000625

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









