Design, Synthesis, and Evaluation of New Colorimetric Chemosensors Containing Quinazolinones Moiety for some Cations Detection in an Aqueous Medium and Biological Sample
DOI:
https://doi.org/10.29356/jmcs.v65i3.1500Keywords:
Chemosensor, quinazolinone, copper, cadmium, mercuryAbstract
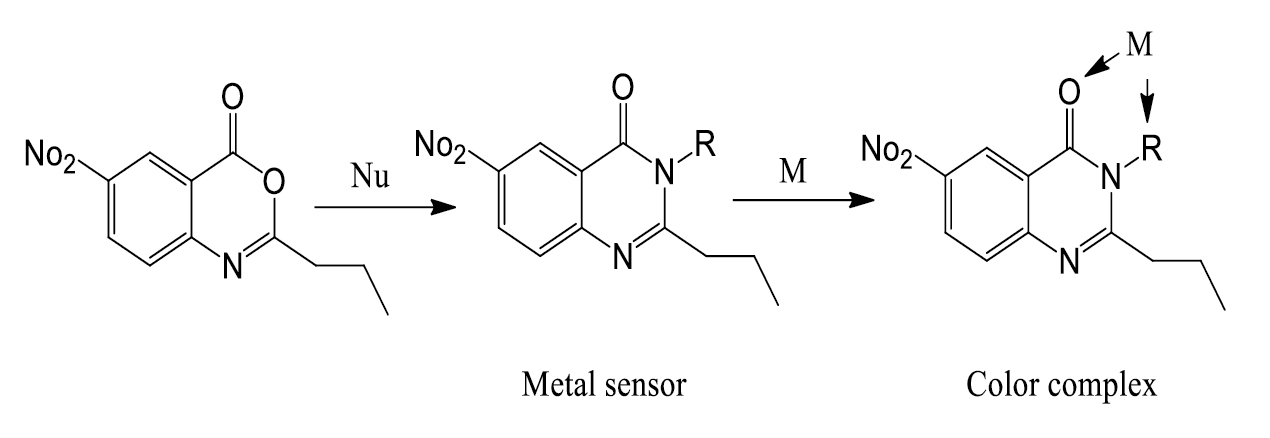
Abstract. The current project deals with designing and synthesizing of colorimetric chemosensors to detect the cations in the aqueous medium and biological sample. To achieve this goal a new series of quinazolinone derivatives were synthesized via reaction of the novel 6-nitro-2-propyl-4H-benzo[d][1,3]oxazin-4-one (3) with selected nitrogen nucleophiles, namely, formamide, hydrazine hydrate, hydroxylamine hydrochloride, O-phenylendiamine, O-aminophenol and O-aminothiophenol, urea and/or thiourea. Structures of the new compounds have been investigated depending on their spectral data (IR, 1H NMR, 13C NMR and MS) and elemental analyses. Some of the newly synthesized products exhibited a significant response as chemosensors for some cations detection. The synthesized chemosensors 11a and 11b showed high-selectivity and specificity towards cooper (CuII) and mercury (HgII) cations detection through exhibiting colormetric responses. Chemosensors 7 and 10b showed high selectivity toward cadmium (CdII) cation, whilst other examined compounds (9b, c, 10a, 12, 13, and 14) did not exhibit colorimetric response in all cation's samples.
Resumen. En el presente proyecto se diseñan y sintetizan quimiosensores colorimétricos para detectar los cationes en el medio acuoso y en la muestra biológica. Para lograr este objetivo se sintetizó una nueva serie de derivados de quinazolinona mediante la reacción de la 6-nitro-2-propil-4H-benzo[d][1,3]oxazin-4-ona (3) con nucleófilos nitrogenados seleccionados, a saber, formamida, hidrato de hidracina, clorhidrato de hidroxilamina, O-fenilendiamina, O-aminofenol y O-aminotiofenol, urea y/o tiourea. Las estructuras de los nuevos compuestos se han comprobado en función de sus datos espectrales (IR, 1H NMR, 13C NMR y MS) y de los análisis elementales. Algunos de los nuevos productos sintetizados mostraron una respuesta significativa como quimiosensores para la detección de algunos cationes. Los quimiosensores sintetizados 11a y 11b mostraron una alta selectividad y especificidad hacia la detección de los cationes cobre (Cu II) y mercurio (Hg II) al mostrar respuestas colormétricas. Los quimiosensores 7 y 10b mostraron una alta selectividad hacia el catión cadmio (Cd II), mientras que otros compuestos examinados (9b, c, 10a, 12, 13 y 14) no mostraron respuesta colorimétrica con los cationes investigados.
Downloads
References
Anu Kundu, P.S.; Hariharan, K. P.; Savarimuthu, P. A. Sensors and Actuators B. 2015, 206, 524-530.
Kevin, J. B.; Colin, L. M.; Ashley, I. B. Nat. Rev. Drug Discov. 2004, 3, 205-2014.
Robert, R. L.; Alfred, M. B.; Harry, A. R.; Jean, P.B. CLIN. CHEM. 1994, 40, 1391-1394.
Ullah, I. K. ; Rahim, M. ; Haris, M. J. Chem. Soc. Pak. 2016, 38, 177-185.
Ivan, S. ; Irina, K. ; Trajce, S.; Juli, J. Microchem. J. 2008, 89,42-50.
Leermakers, M. W.; Baeyens, P.; Horvat, M. Trends Anal.Chem. 2005, 24, 383-392.
Weiying, L.; Lin, Y.; Wen, T.; Jianbo, F.; Lingliang, L. Chem. Eur. J. 2009, 15, 1030 -1035.
Takunori, K.; Seiji, N.; Masatoshi, M. Anal. Sci. 1990, 6, 623-626.
Paramjit, K.; and Divya, S. Dyes Pigm. 2011, 88, 296-300.
Ha, N.; Wa, Wen. ; X. R.; Jong, S. K.; Juyoung, Y. Chem. Soc. Rev. 2012, 41, 3210-3244.
Meng, Li.; Hai-Yan, Lu. ; Rui-Li, Liu. ; Jun-Dao, C.; Chuan-Feng, C. J. Org. Chem. 2012, 77, 3670-3673.
Umesh, F. ; Anu, S. Suban, K. S. ; Narinder, S. ; Ratnamala, B. ; Anil, K. RSC Adv. 2014, 4, 39639-39644.
Xue-Jiao, B.; Jing, R.; Jia, Z.; Zhi-Bin, S. Heterocycl. Commun. 2018, 24,135–139.
Chunliang, L. ; Zhaochao, X. ; Jingnan, C. ; Rong, Z. ; Xuhong, Q. J. Org. Chem. 2007, 72, 3554-3557.
Maher, A. E. ; Khalid, M. D. ; Sameh, A. R. ; Fakhry, A. E. Pharmaceuticals. 2011, 4, 1032-1051.
Ailin, Y.; Chunling, Z.; Zhengyu, Z.; Lu, Y.; Chao, L.; Haibo, W. Fluoresce. 2014, 24, 557-561.
Pravin, N. B.; Pranila, B. T.; Ganapati, S. S. Dyes Pigm. 2016, 134, 276-284.
Ayman, M. F. E.; Mohamed, M. H.; Mohamed, A. Der Pharma Chem. 2011, 3, 1-12.
Maher, A. E.; Mohamed, E. A.; Jehan, M. M. J. Heterocyclic Chem. 2016, 53, 95-101.
Ghorab, M.M.; Hassan, A.Y. Phosphorus, Sulfur and Silicon. 1998, 141, 251-261.
Joule, A. ; Mills, K. ; Smith, F. Heterocyclic Chemistry Springer, 3rd Ed., 1995.
Paul, G. ; Tinku, B. ; William, P. M. ; Alexander, J. M. ; George, C. P. ; James D. ; Shauna, B. ; Ashley, M. D. J. Med. Chem. 2006, 49, 684-692.
Ashok, K.; Chatrasal, S. R. E. J. Med. Chem. 2011, 44, 83-90.
Venkatadri, T.; Darshak, R. T. Anal. Chim. Acta. 2017, 972, 81-93.
Lu, C.; Xu, Z.; Cui, J.; Zhang, R.; Qian, X. J. Org. Chem. 2007, 72, 3554-3557.
- Wiberg, K. B.; Lampman, G. M.; Ciula, R. P.; Connor, D. S.; Schertler, P.; Lavanish, J. Tetrahedron 1965, 21, 2749-2796.
Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









