Multi-walled Carbon Nanotubes Synthesis by Arc Discharge Method in a Glass Chamber
DOI:
https://doi.org/10.29356/jmcs.v65i4.1486Keywords:
CNT, arc discharge, catalyst, MWCNTs, hybridizationAbstract
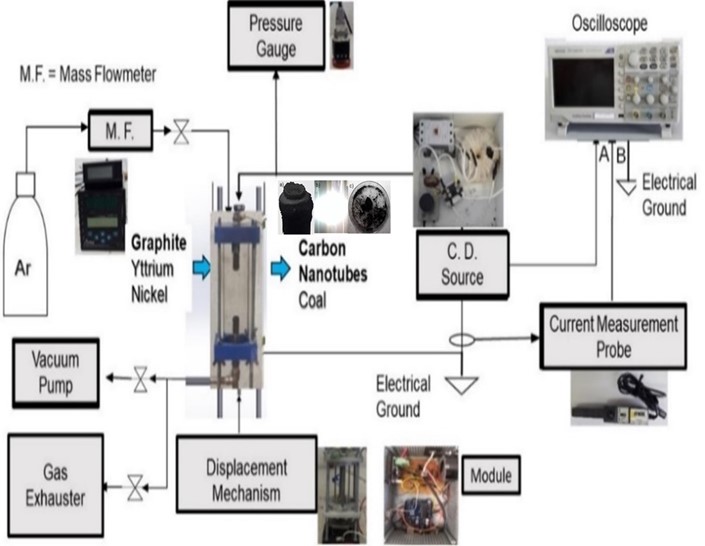
Abstract. In the present paper, carbon nanotubes (CNTs) were synthesized by arc discharge method by vaporizing graphite rods in the presence of Ni and a Ni/Y mixture as catalysts for subsequent use in electrical energy storage and conversion devices. CNTs synthesis was carried out in a cylindrical glass reactor applying a controlled Argon flow of 1.43 cm3/min and a chamber pressure of 39 kPa. Carbon powder was collected from the reactor following chemical treatment with HCl solution at 1 M to remove the metallic impurities. Morphology obtained by scanning electron microscopy (SEM) and transmission electron microscopy (TEM) showed multi-walled nanotubes (MWCNTs) with amorphous carbon particles attached to their surface. Fourier transform infrared (FT-IR) spectra presented bands at 1550 and 1200 cm–1 corresponding to the C=C bond characteristic to the CNTs skeleton; these bands were not present in pristine graphite. Electromagnetic absorbance was observed using ultraviolet-visible spectroscopy (UV-Vis) showing peaks at 204 and 256 nm related to sp2 hybridization characteristic for MWCNTs.
Resumen. En el presente trabajo se sintetizaron nanotubos de carbono (NTCs) por el método de descarga de arco mediante la vaporización de barras de grafito en presencia de Ni y una mezcla de Ni/Y como catalizadores para su posterior uso en dispositivos de almacenamiento y conversión de energía eléctrica. La síntesis de NTCs se realizó en un reactor cilíndrico de vidrio aplicando un flujo controlado de Argón de 1.43 cm3/min y una presión de cámara de 39 kPa. El polvo de carbón se recolectó del reactor y se trató químicamente con una solución de HCl a 1 M para eliminar las impurezas metálicas. La morfología obtenida por microscopía electrónica de barrido (MEB) y microscopía electrónica de transmisión (MET) mostró nanotubos de paredes múltiples (NTCPMs) con partículas de carbón amorfo adheridas a su superficie. Los espectros de infrarrojo transformada de Fourier (FT-IR) mostraron bandas en 1550 y 1200 cm–1 que corresponden al enlace C=C característico de los NTCs; estas bandas no estuvieron presentes en el grafito prístino. La absorbancia electromagnética se observó mediante espectroscopia ultravioleta-visible (UV-Vis) mostrando picos a 204 y 256 nm relacionadas con la hibridación sp2 característica de los NTCPMs.
Downloads
References
Zou, J.; Zhang, Q. In Carbon Nanotubes and Their Applications, Ed. CRC Press. USA. 2012, 1-27. DOI: https://doi.org/10.1201/b11989
Eatemadi, A.; Daraee, H.; Karimkhanloo. H.; Kouh, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpou, Y.; Joo, S. W. Nanoscale Res. Lett. 2014, 9, 1-31. DOI: http://doi:10.1186/1556-276X-9-393. DOI: https://doi.org/10.1186/1556-276X-9-393
Candelaria, S. L.; Shao, Y.; Zhou, W.; Li, X.; Xiao, J.; Zhang, J.-G.; Wang, Y.; Liu, J.; Li, J.; Cao, G. Nano Energy. 2012, 1, 195–220. DOI: http://doi:10.1016/j.nanoen.2011.11.006 DOI: https://doi.org/10.1016/j.nanoen.2011.11.006
Chen, T.; Dai, L. Mater. Today. 2013, 16, 272-280. DOI: http://dx.doi.org/10.1016/j.mattod.2013.07.002 DOI: https://doi.org/10.1016/j.mattod.2013.07.002
Hanaei, H,; Assadi, M. K.; Saidur, R. Renewable Sustainable Energy Rev, 2016, 59, 620–635 DOI: http://dx.doi.org/10.1016/j.rser.2016.01.017 DOI: https://doi.org/10.1016/j.rser.2016.01.017
Paradise, M.; Goswami, T. Mater. Des. 2007, 28, 1477-1489. DOI: http://doi:10.1016/j.matdes.2006.03.008 DOI: https://doi.org/10.1016/j.matdes.2006.03.008
Liu, J.; Dai, L. Carbon Nanomater. Adv. Energy Syst., Ed. John Wiley & Sons, Inc. USA. 2015. 165-189.
Zhang, L. L.; Zhao, X. S. Chem. Soc. Rev. 2009, 38, 2520-2531. DOI: http://DOI:10.1039/b813846j DOI: https://doi.org/10.1039/b813846j
Sharma, R.; Sharma, A. K.; Sharma, V. Cogent Engineering. 2015, 2, 1-10. DOI: http://dx.doi.org/10.1080/23311916.2015.1094017 DOI: https://doi.org/10.1080/23311916.2015.1094017
He, B.; Tang, Q.; Luo, J.; Li, Q.; Chen, X.; Cai, H. J. Power Sources. 2014, 170-177. DOI: http://dx.doi.org/10.1016/j.jpowsour.2014.01.072 DOI: https://doi.org/10.1016/j.jpowsour.2014.01.072
Snook, G. A.; Kao, P.; Best, A. S. J. Power Sources. 2011, 196, 1–12. DOI: http://doi:10.1016/j.jpowsour.2010.06.084 DOI: https://doi.org/10.1016/j.jpowsour.2010.06.084
Hosseini, T. ; Kouklin, N. In Carbon Nanotubes: Curr. Prog. Their Polym. Compos., Ed. InTech, Croatia. 2016, 95-123. DOI: http://dx.doi.org/10.5772/62692 DOI: https://doi.org/10.5772/62692
Dai, H. Acc. Chem. Res. 2002, 35, 1035-1044. DOI: https://doi.org/10.1021/ar0101640
Ostrogorsky, A. G.; Marín, C. Heat Mass Transfer. 2006, 42, 470-477. DOI: http://DOI:10.1007/s00231-005-0644-7 DOI: https://doi.org/10.1007/s00231-005-0644-7
Levchenko, I.; Keidar, M.; Xu, S. Kersten, H.; Ostrikov, K. J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct.-Process. Meas. Phenom. 2013, 31, 1-16. DOI: http://dx.doi.org/10.1116/1.4821635 DOI: https://doi.org/10.1116/1.4821635
Mohammad, M. I.; Moosa, A. A.; Potgieter, J. H.; Ismael, M. K. ISRN Nanomater. 2013, 1-7. DOI: http://dx.doi.org/10.1155/2013/785160 DOI: https://doi.org/10.1155/2013/785160
Tarasov, B. P.; Muradyan, V. E.; Shul’ga, Y. M.; Krinichnaya, E. P.; Kuyunko, N. S.; Efimov, O. N.; Obraztsova, E. D.; Schur, D. V.; Maehlen, J. P.; Yartys, V. A.; Lai H.-J. Carbon. 2003, 41, 1357–1364. DOI: https://doi.org/10.1016/S0008-6223(03)00060-5
Mamun, A. A.; Ahmed, Y. M.; Muyibi, S. A.; Al-Khatib, M. F. R.; Jameel, A. T.; AlSaadi M.A. Arabian J. Chem. 2016, 9, 532–536. DOI: http://dx.doi.org/10.1016/j.arabjc.2013.09.001 DOI: https://doi.org/10.1016/j.arabjc.2013.09.001
Hou, P.-X.; Liu C.; Cheng H.-M. Carbon. 2008, 46, 2003–2025. DOI: http://doi:10.1016/j.carbon.2008.09.009 DOI: https://doi.org/10.1016/j.carbon.2008.09.009
Wen, L.; Jong?Beom, B.; Liming, D. In Carbon Nanomater. Adv. Energy Syst., Ed. John Wiley & Sons, Inc. USA. 2015, XVII-XVIII.
Lian, Y.; Maeda, V.; Wakahara, V.; Akasaka, T.; Kazaoui, V.; Minami, V.; Shimizu, V.; Choi, V.; Tokumoto, V. J. Phys. Chem. B. 2004, 108, 8848-8854. DOI: https://doi.org/10.1021/jp049368z
Prasek, J.; Drbohlavova, J.; Chomoucka, J.; Hubalek, J.; Jasek, O.; Adam, V.; Kizek, R. J. Mater. Chem. 2011, 21, 15872-15884. DOI: 10.1039/c1jm12254a DOI: https://doi.org/10.1039/c1jm12254a
Prakash, D.; Amente, C.; Dharamvir. K.; Singh, B.; Singh, R.; Shaaban, E. R.; Al-Douri, Y.; Khenata, R.; Darroudi, M.; Verma, K.D. Ceram. Int. 2016, 42, 5600-5606. DOI: http://dx.doi.org/10.1016/j.ceramint.2015.11.074 DOI: https://doi.org/10.1016/j.ceramint.2015.11.074
Zhang, J.; Wu, C.; Hou, K.; Huang, M.; Guan, L. Int. J. Hydrogen Energy, 2018, 43 , 15687- 5692. DOI: https://doi.org/ 10.1016/j.ijhydene.2018.07.048 DOI: https://doi.org/10.1016/j.ijhydene.2018.07.048
Pifferi, V.; Cappelletti, G.; Di Bari C.; Meroni, D.; Spadavecchia, F.; Falciola, L. Electrochim. Acta, 2014, 146, 403–410. DOI: http://dx.doi.org/10.1016/j.electacta.2014.09.099 DOI: https://doi.org/10.1016/j.electacta.2014.09.099
Das, R.; Ali, M. E.; Hamid, S. B. A. J. Nanomater. 2014, 2014, 1-9. DOI: http://dx.doi.org/10.1155/2014/945172 DOI: https://doi.org/10.1155/2014/945172
Wulan, P. P. D. K.; Permana, G.; Putri, W. A. AIP Conf. Proc. 2020, 2255, 0600141-0600146. DOI : https://doi.org/10.1063/5.0014074
Raniszewski, G.; Wiak, S. ¸ Pietrzak, L.; Szymanski, L.; Kolacinski, Z. Nanomaterials. 2017, 7, 1-12. DOI: doi:10.3390/nano7030050 DOI: https://doi.org/10.3390/nano7030050
Roslan, M. S.; Chaudhary, K. T.; Doylend, N. J. Saudi Chem. Soc. 2019, 23, 171–181. DOI: https://doi.org/10.1016/j.jscs.2018.06.003
Yermagambet, B. T.; Kazankapova, M. K.; Kassenova1, Z. M.; Nauryzbayeva, A. T. Nats Akad. Nauk. Resp. Kaz., Ser. Khim. Tekhnol. 2020, 5, 126-133. DOI : https://doi.org/10.32014/2020.2518-1491.89
Kannan, M., in Fundamentals and applications of Nanotechnology, Subramanian K. S.; Janavi G. J.; Marimuthu S., Ed. Astral, 2018, 81-92.
Brydson, R.; Brown, A.; Benning, L. G. Rev. Mineral. Geochem. 2014, 78. 219-269. DOI: https://doi.org/10.2138/rmg.2014.78.6
Tepale, A.; Moreno, H.; Hernandez, C. Congr. Int. en Ing. Electrónica. Mem. ELECTRO. 2019, 41, 81-85.
Cotul, U.; Parmak, E. D. S.; Kaykilarli, C.; Saray, O.; Colak, O.; Uzunsoy; D. Acta Phys. Pol., A. 2018, 134, 289-291. DOI: http://DOI:10.12693/APhysPolA.134.289 DOI: https://doi.org/10.12693/APhysPolA.134.289
Abdel-Salam, M.; Burke, R. Arabian J. Chem. 2017, 921-927. DOI: http://dx.doi.org/10.1016/j.arabjc.2012.12.028 DOI: https://doi.org/10.1016/j.arabjc.2012.12.028
Xu, S.; Liu, J.; Li Q. Constr. Build. Mater. 2015, 76, 16–23. DOI: http://dx.doi.org/10.1016/j.conbuildmat.2014.11.049 DOI: https://doi.org/10.1016/j.conbuildmat.2014.11.049
Feng, L.; Li, K-Z.; Xue, B. Mater. Lett. 2017, 187, 158–161. DOI: http://dx.doi.org/10.1016/j.matlet.2016.10.067 DOI: https://doi.org/10.1016/j.matlet.2016.10.067
Toma, S.; Asaka, K.; Irita, M.; Saito, Y. Surf. Interface Anal. 2019, 131-135. DOI: http://DOI:10.1002/sia.6590 DOI: https://doi.org/10.1002/sia.6590
Kumar, S.; Nehra, M.; Kedia, Prog. Energy Combust. Sci. 2017, 1-35. DOI: http://dx.doi.org/10.1016/j.pecs.2017.10.005 0360-1285/ DOI: https://doi.org/10.1016/j.pecs.2017.10.005
García-Ruiz, D. L.; Granados-Martínez, F. G.; Gutiérrez-García, C. J.; Ambriz-Torres, J.M.; Contreras-Navarrete, J.J.; Flores-Ramírez, N.; García-González, L.; Zamora-Peredo, L.; Mondragón-Sánchez, M. L.; Domratcheva-Lvova L. Rev. Mex. Ing. Quim. 2019, 18, 659-671. DOI: https://doi.org/10.24275/uam/izt/dcbi/revmexingquim/2019v18n2/GarciaR
?ucureanu, V.; Matei, A.; Avram, A. M. Crit. Rev. Anal. Chem. 2016, 46, 1547-6510. DOI: http://dx.doi.org/10.1080/10408347.2016.1157013 DOI: https://doi.org/10.1080/10408347.2016.1157013
Rance, G. A.; Marsh, D. H.; Nicholas, R. J.; Khlobystov , A. N. Chem. Phys. Lett. 2010, 493, 19–23. DOI: doi:10.1016/j.cplett.2010.05.012 DOI: https://doi.org/10.1016/j.cplett.2010.05.012
Grossiord, N.; Regev, O.; Loos, J.; Meuldijk, J.; Koning, C. E. Anal. Chem. 2005, 77, 5135-5139. DOI: https://doi.org/10.1021/ac050358j
Alafogianni, P.; Dassios, K.; Farmaki, S.; Antiohos, S. K.; Matikas, T. E.; Barkoula, N.-M. Colloids Surf., A. 2016, 495, 118–124. DOI: http://dx.doi.org/10.1016/j.colsurfa.2016.01.053 DOI: https://doi.org/10.1016/j.colsurfa.2016.01.053
Njuguna, J.; Vanli, O. A.; Liang, R. J. Spectrosc. 2015, 1–11. DOI: http://dx.doi.org/10.1155/2015/463156 DOI: https://doi.org/10.1155/2015/463156

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









