Physicochemical and Biopharmaceutical Evaluation of Casiopeina III-Ea, a New Compound with Antineoplastic Activity
DOI:
https://doi.org/10.29356/jmcs.v65i3.1471Keywords:
Casiopeina III-Ea, physicochemical properties, IC50, MDCK cells, copperAbstract
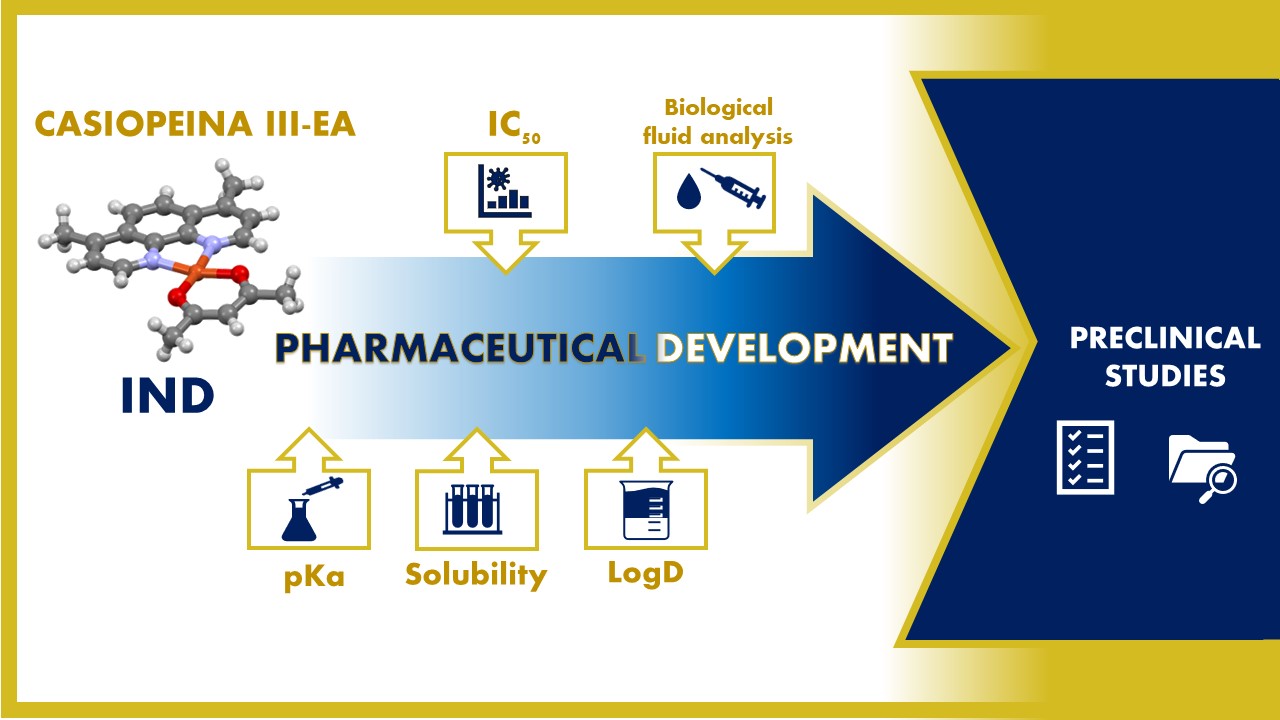
Abstract. In the present work, the physicochemical (pka, intrinsic solubility, Log D distribution coefficient) and biopharmaceutical characterization (IC50 studies in MDCK cells) of Casiopeina III-Ea, a Cu (II) mixed chelate compound, was carried out. These parameters will tell us about the behaviour of Casiopeina III-Ea, as a new antineoplastic agent, in physiological media. This basic research of the Faculty of Chemistry of the UNAM, is directed towards the maturation of a technology, which consists of the pharmaceutical development of an antineoplastic copper coordination compound Casiopeina III-Ea.
Resumen. En el presente trabajo se realizó la caracterización fisicoquímica (pka, solubilidad intrínseca, coeficiente de distribución Log D) y biofarmacéutica (estudios IC50 en células MDCK) de Casiopeina III-Ea, un nuevo compuesto quelato mixto de cobre (II). Estos parámetros nos dirán mucho sobre el comportamiento de Casiopeina III-Ea, como un nuevo agente antineoplásico, en medios fisiológicos. Esta investigación básica de la Facultad de Química de la UNAM, está dirigida hacia la maduración de una tecnología, que consiste en el desarrollo farmacéutico de un compuesto de coordinación antineoplásico de cobre Casiopeina III-Ea.
Downloads
References
Ruiz-Azuara, L.; Bravo-Gómez, M.E. Curr. Med. Chem. 2010, 17, 3606-3615. DOI: 10.2174/092986710793213751
Gutiérrez, A. G.; Vázquez-Aguirre, A.; García-Ramos, J.C.; Flores-Alamo, M.; Hernández-Lemus, E.; Ruiz-Azuara, L.; Mejía, C. J. Inorg. Biochem. 2013, 126, 17-25. DOI: 10.1016/j.jinorgbio.2013.05.001
Vidal, L.M.; Pimentel, E.; Cruces, M.P.; Hernández, S.; Ruiz-Azuara, L. J. Toxicol. Environ. Eealth, part A. 2017, 80, 365-373. DOI: 10.1080/15287394.2017.1326072
Rivero-Müller, R.; De Vizcaya-Ruiz, A.; Plant, N.; Ruiz-Azuara, L.; Dobrota M. Chem. Biol. Interact. 2007, 165, 189–199. DOI: 10.1016/j.cbi.2006.12.002
Alemón-Medina, R.; Brena-Valle, M.; Muñoz-Sánchez, J. L.; Gracia-Mora, M. I.; Ruiz-Azuara, L. Cancer Chemother. Pharmacol. 2007, 60, 219-228. DOI: 10.1007/s00280-006-0364-9
Mejía, C.; Ruiz-Azuara, L. Pathol. Oncol. Res. 2008, 14, 467-472. DOI: 10.1007/s12253-008-9060-x
Trejo-Solís, C.; Palencia, G.; Zúñiga, S.; Rodríguez-Ropon, A.; Osorio-Rico, L.; Sanchez Torres, L.; Gracia-Mora, I.; Marquez-Rosado, L.; Sánchez, A.; Moreno-García, M. E.; Cruz, A.; Bravo-Gómez, M. E.; Ruiz-Ramírez, L.; Rodríguez-Enriquez, S.; Sotelo, J. Neoplasia. 2005, 7, 563–574. DOI: 10.1593/neo.04607
Bravo-Gómez, M. E.; García-Ramos, J. C.; Gracia-Mora, I.; Ruiz-Azuara, L. J. Inorg. Biochem. 2009, 103, 299-309. DOI: 10.1016/j.jinorgbio.2008.10.006
Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T., Bokesch, H.; Kenney, S.; Boyd, M.R. J. Natl. Cancer Inst. 1990, 82, 1107-1112. DOI: 10.1093/jnci/82.13.1107
Vértiz, G.; García-Ortuño, L. E.; Bernal, J. P.; Bravo-Gómez, M. E.; Lounejeva, E.; Huerta, A.; Ruiz-Azuara, L. Fundam. Clin. Pharmacol. 2014, 28,78-87. DOI: 10.1111/j.1472-8206.2012. 01075.x
García-Ramos, J. C.; Galindo-Murillo, R.; Tovar-Tovar, A.; Alonso-Saenz, A. L.; Gómez-Vidales, V.; Flores-Alamo, M.; Ortiz-Frade, L.; Cortes-Guzmán, F.; Moreno-Esparza, R.; Campero, A.; Ruiz-Azuara, L. Chem. Eur. J. 2014, 20, 13730-13741. DOI: 10.1002/chem.201402775
Chavez-Gonzalez, A.; Centeno-Llanosa, S.; Moreno-Lorenzana, D.; Sandoval-Esquivela, M. A.; Aviles-Vazquez, S.; Bravo-Gómez, M. E.; Ruiz-Azuara, L.; Ayala-Sanchez, M.; Torres-Martineze, H.; Mayania, H. Leuk. Res. 2017, 52, 8-19. DOI: https://doi.org/10.1016/j.leukres.2016.11.001
Guidance for Industry Statistical Approaches to Establishing Bioequivalence, U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER), 2001, 1-48. https://ntrl.ntis.gov/NTRL/dashboard/searchResults/titleDetail/PB2010104191.xhtml
Reyes, L.; Fuentes-Noriega, I.; Ruiz-Ramírez, L.; Macías, L. J. Chromatogr. B. 2003, 791, 111-116. DOI: 10.1016/s1570-0232(03)00226-5
Fuentes-Noriega, I.; Ruiz-Ramírez, L.; Tovar Tovar, A.; Rico-Morales, H.; Gracia-Mora, I. J. Chromatogr. B. 2002, 772, 115-121. https://doi.org/10.1016/S1570-0232(02)00064-8
Cañas, A. R. C., Fuentes-Noriega, I.; Ruiz-Azuara, L. J. Bioanal. Biomed. 2010, 2, 28-34. DOI: 10.4172/1948-593X.1000018
Takács-Nova´k, K.; Avdeef, A. J. Pharm. Biomed. Anal. 1996, 14, 1405-1413. DOI: https://doi.org/10.1016/0731-7085(96)01773-6
Yalkowskv, S.H.; Valvani, S.C.; Mackay, D. Residue Rev. 1983, 85, 43-55. DOI:10.1007/978-1-4612-5462-1_5.
Comer, J.E.A. High throughput measurement of log D and pKa. In P. Artursson, H. Lennernas & H. van de Waterbeemd (Eds.) Methods and principles in medicinal chemistry 18 , Weinheim Wilet-VCH, 2003, 21-45.
Riss, T.; Niles, A.; Moravec, R.; Karassina N.; Vidugiriene, J. Sciences 2019, 1-15. https://www.ncbi.nlm.nih.gov/books/NBK540958/(visited January 23, 2021).
Niles, A. L.; Moravec, R. A.; Riss, T.L. Expert Opin Drug Discov. 2008, 3, 655-669.DOI: 10.1517/17460441.3.6.655
Diaz, J. Casiopeinas stability at different pH?s. Bachelor thesis. Faculty of Chemistry. UNAM. In preparation.
Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









