Synthesis of New Hispolon Derived Pyrazole Sulfonamides for Possible Antitubercular and Antimicrobial Agents
DOI:
https://doi.org/10.29356/jmcs.v65i2.1458Keywords:
Hispolon, sulfonamides, hispolon pyrazole, antimicrobial activity, pyrazole sulfonamides, anti TBAbstract
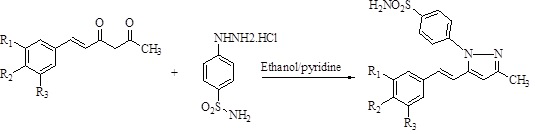
Abstract. A series of 10 new hispolonpyrazole sulfonamides were designed and synthesized using hispolons and 4-sulfonamide phenylhydrazine hydrochloride with better yields. The synthesized pyrazole sulfonamides were screened for anti-TB, anti-bacterial, and anti-fungal activities to compare the role sulfonamide moiety. Among them, 3a and 3b showed selective potent anti-tubercular nature (MIC 6.25µg/mL). Further, the antimicrobial studies of the compounds showed only compound 3b with a good inhibition zone on Staphylococcus aureus among other bacteria and fungi studied.
Resumen.Se prepararon 10 nuevas hispolon-pirazol-suklfonamidas con buenos rendimientos haciendo reaccionar hipolonas y el clorhidrato de 4-sulfonamidil-fenílhidrazina. Los productos obtenidos se probaron contra cepas de hongos y bacterias con especial interés en la tuberculosis, encontrando algunos derivados con actividades del orden de MIC 6.25µg/mL.
Downloads
References
Hewlings, S. J.; Kalman, D. S. Foods 2017, 6, 1-11.
Ali, NA.; Ludtke, J.; Pilgrim, H.; Lindequist, U. Pharmazie. 1996, 51, 667.
Yang, L. Y.; Shen, S. C.; Cheng, K. T.; Subbaraju, G. V.; Chien, C. C.; Chen, Y. C. J. Ethnopharmacol. 2014, 156, 61-72.
Huang, G. J.; Deng, J. S.; Chiu, C. S.; Liao, J. C.; Hsieh, W. T.; Sheu, M. J.; Wu, C. H. eCAM2012, Article ID: 480714 (1-12).
Wang, J.; Hu, F.; Luo, Y.; Luo, H.; Huang, N.; Cheng, F.; Deng, Z.; Deng, W.; Zou, K. Fitoterapia 2014, 95, 93-101.
Balaji, N. V.; Babu, B. H.; Subbaraju, G. V.; Nagasree, K. P.; Kumar, M. M. K. Bioorg. Med. Chem. Lett. 2017, 27, 11-21.
Chen, T.; Wong, Y. S. J. Agric. Food Chem. 2008, 56, 10574-10581.
Lu, T. L.; Huang, G. J.; Lu, T. J.; Wu, J. B.; Wu, C. H.; Yang, T. C.; Iizuka, A.; Chen, Y. F. Food Chem. Toxicol. 2009, 47, 2013-2021.
Hsieh, M.J.; Chien, S. Y.; Chou, Y. E.; Chen, C. J.; Chen, J.; Chen, M. K. Phytomedicine 2014, 21, 1746-1752.
Wu, Q.; Kang, Y.; Zhang, H.; Wang, H.; Liu, Y.; Wang, J. Biochem. Biophys. Res. Commun. 2014, 53, 385-391.
Ravindran, J.; Subbaraju, G. V.; Ramani, M. V.; Sung, B.; Agarwal, B. B. Biochem. Pharmacol. 2010, 79, 1658-1666.
Balaji, N. V.; Ramani, M. V.; Vanisree, M.; Crystal, L.; Theophilus, J. G.; Nosa, O.; Subbaraju, G. V.; Amit, K. T. Bioorg. Med. Chem. 2015, 23, 2148-2158.
Wu, J. P.; Tsai, C. C.; Yeh, Y. L.; Lin, Y. M.; Lin, C. C.; Day, C. H.; Shen, C. Y. Padma, V. V. Pan, L. F.; Huang, C. Y. Evid. Based Complementary Altern. Med. 2015, 603529. PMID 26339266 DOI: 10.1155/2015/603529.
Balaji, N. V.; Babu, B. H.; Rao, V. U.; Subbaraju, G. V.; Nagasree, K. P.; Kumar, M. M. K. Curr. Top. Med. Chem. 2019, 19, 662-682.
Pareek, A.; Priyanka, R.; Kishore, D. Int. J. Pharm. Biosci. 2013, 4, 812-820.
Lesch, J. E. The first miracle drugs: how sulfa drugs transformed medicine, Oxford University Press. New York, 2007, 364.
Osadebe, P. O.; Odoh, U.E.; Uzor, P. Brit. J. Med. Med. Res. 2015, 5, 134-139.
Bombardier, C.; Laine, L.; Reicin, A.; Shapiro, D.; Burgos-Vargas, R.; Davis, B.; Day, R.; Ferraz, M. B.; Hawkey, C. J.; Hochberg, M. C.; Kvien, T. K.; Schnitzer, T. J. N. Engl. J. Med. 2000, 343, 1520-1528.
Wood, W.B. J. Exp Med. 1942, 75, 369-381.
Finch, R.; Greenwood, D.; Whitley, R.; Norrby, S. R. 9th ed., Elsevier. 2010, ISBN 978-0-7020-4064-1.
Rambabu, A.; Surender, S.; Babu, A. V.; Ahsan, Md. J.; Babu, B. H. Med. Chem. Res. 2018, 27, 1690-1704.
Ramana, M. B.; Vijaya, K.; Reddy, O. S.; Murthy, B. S. N.; Babu, B. H. Chem. Afri. 2019, 2, 15-20.
Diego G. G.; Agustina de la I.; Nina L.;, Peter J. T.; Héctor R. M.; Guillermo R. L.; Eur J Med Chem. 2017, 125, 842–852.
Downloads
Additional Files
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









