Understanding the Reactivity of C-Cyclopropyl-N-Methylnitrone Participating in [3+2] Cycloaddition Reactions Towards Styrene with a Molecular Electron Density Theory Perspective
DOI:
https://doi.org/10.29356/jmcs.v65i1.1437Keywords:
Electron localization function, isoxazolidines, [3 2] cycloaddition reactions, molecular electron density theoryAbstract
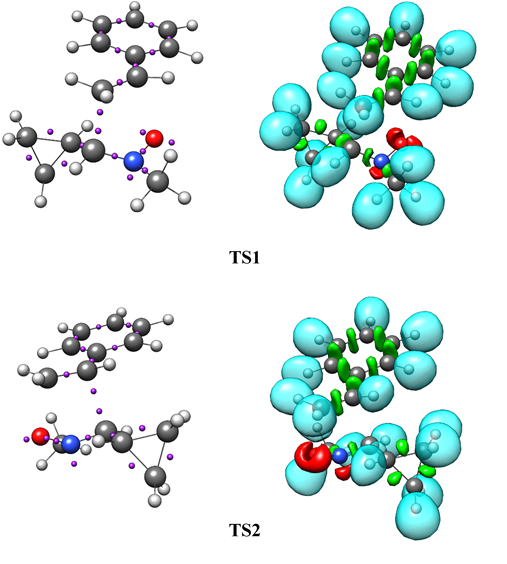
Abstract. The [3+2] cycloaddition (32CA) reactions of C-cyclopropyl-N-methylnitrone 1 with styrene 2 have been studied within molecular electron density theory (MEDT) at the B3LYP/6-311++G(d,p) level of theory. These zwitterionic type 32CA reactions occur through a one-step mechanism. The 32CA reactions undergo four stereo- and regioisomeric reaction paths to form four different products, 3, 4, 5 and 6. Analysis of the conceptual density functional theory (CDFT) indices predict the global electronic flux from the strong nucleophilic nitrone 1 to the styrene 2. These 32CA reactions are endergonic with reactions Gibbs free energies between 2.83 and 7.39 kcal.mol-1 in the gas phase. The 32CA reaction leading to the formation of cycloadduct 3 presents the lowest activation enthalpy than the other paths due to a slightly increase in polar character evident from the global electron density transfer (GEDT) at the transition states and along the reaction path. The bonding evolution theory (BET) study suggests that these 32CA reactions occur through the coupling of pseudoradical centers and the formation of new C-C and C-O covalent bonds has not been started in the transition states.
Resumen. Las reacciones de cicloadición [3+2], denotadas como 32CA, entre C-ciclopropil-N-metilnitrona 1y estireno 2se estudian mediante la teoría de la densidad electrónica molecular (MEDT) utilizando el nivel de teoría B3LYP/6-311++G(d,p). Estas reacciones 32CA de tipo zwiteriónicasse llevan por un mecanimso de un paso. Las reacciones 32CA ocurren a través de cuatro caminos estereo y regioisoméricos de reacción formando cuatro diferentes productos 3, 4, 5y 6. El análisis de los índices de la teoría conceptual de funcionales de la densidad (CDFT) predice un flujo electrónico global de la nitrona 1, un nucleófilo fuerte, al estireno 2. En fase gaseosa,estas reacciones 32CA son endergónicas con energías libres de Gibbs entre 2.83 y 7.39 kcal.mol-1. La reacción 32CA que lleva a la formación del cicloaducto 3tiene la menor entalpía de activación de las cuatro consideradas y esto se debe a un ligero incremento en el carácter polar que es evidente con la transferencia global de densidad electrónica (GEDT) en los estados de transición y a lo largo de los caminos de reacción. El esudio mediante la teoría de evolución del enlace (BET) sugiere que estas reacciones 32CA ocurren mediante el acoplamiento de centros pseudoradicalesy la formación de nuevos enlaces covalentes C-C y C-O que no han iniciado en el estado de transición.
Downloads
References
Mohammad-Salim, H. A.; Abdallah, H. H.; Maiyelvaganan, K. R.; Prakash, M.; Hochlaf, M. Theor. Chem. Acc. 2020, 139, 19. doi: https://doi.org/10.1007/s00214-019-2542-y
Mohammad-Salim, H. A.; Abdallah, H. H. ARO 2019, 7, 9. doi: https://doi.org/10.14500/aro.10575.
Mohammad Salim, H.; Abdallah?, H. Oriental J. Chem. 2019, 35, 1550-1556. doi: https://doi.org/10.13005/ojc/350512.
Salim, H. A. M.; Abdallah, H. H.; Ramasami, P. IOP Conf. Ser.: Mater. Sci. Eng. 2018, 454, 012049. doi: https://doi.org/10.1088/1757-899x/454/1/012049.
Salim, H. M.; Abdallah, H. H.; Ramasami, P. 2018 International Conference on Advanced Science and Engineering (ICOASE), 9-11 Oct. 2018; 2018; pp 415-419. doi: https://doi.org/10.1109/ICOASE.2018.8548917.
Lauria, A.; Delisi, R.; Mingoia, F.; Terenzi, A.; Martorana, A.; Barone, G.; Almerico, A. M. Eur. J. Org. Chem. 2014, 2014, 3289-3306. doi: https://doi.org/10.1002/ejoc.201301695.
Li, Q.-H.; Wei, L.; Wang, C.-J. J. Am. Chem. Soc. 2014, 136, 8685-8692. doi: https://doi.org/10.1021/ja503309u.
Shevelev, S.; Starosotnikov, A. Chem. Heterocycl. Compd. 2013, 49, 92-115. doi: https://doi.org/10.1007/s10593-013-1233-1.
Mohammad-Salim, H.; Hassan, R.; Abdallah, H. H.; Oftadeh, M. J. Mex. Chem. Soc. 2020, 64. doi: https://doi.org/10.29356/jmcs.v64i2.1111.
Anderson, L. L. Asian J. Org. Chem. 2016, 5, 9-30. doi: https://doi.org/10.1002/ajoc.201500211.
Ríos-Gutiérrez, M.; Darù, A.; Tejero, T.; Domingo, L. R.; Merino, P. Org. Biomol. Chem. 2017, 15, 1618-1627. doi: https://doi.org/10.1039/C6OB02768G
Frederickson, M. Tetrahedron 1997, 53, 403-425. doi: https://doi.org/10.1016/S0040-4020(96)01095-2.
Wanapun, D.; Van Gorp, K.; Mosey, N.; Kerr, M.; Woo, T. Can. J. Chem. 2005, 83, 1752-1767. doi: https://doi.org/10.1139/v05-182.
Domingo, L. R. Molecules 2016, 21, 1319. doi: https://doi.org/10.3390/molecules21101319.
R?os-Gutiérrez, M.; Domingo, L. Eur. J. Org. Chem 2019, 267. doi: https://doi.org/10.1002/ejoc.201800916.
Abbiche, K.; Mohammad-Salim, H.; Salah, M.; Mazoir, N.; Zeroual, A.; El Alaoui El Abdallaoui, H.; El Hammadi, A.; Hilali, M.; Abdallah, H. H.; Hochlaf, M. Theor. Chem. Acc. 2020, 139, 148. doi: https://doi.org/10.1007/s00214-020-02662-4.
Domingo, L. R.; Acharjee, N.; Mohammad-Salim, H. A. Organics 2020, 1, 3-18. doi: https://doi.org/10.3390/org1010002.
Mohammad-Salim, H. A.; Acharjee, N.; Domingo, L. R.; Abdallah, H. H. Int. J. Quantum Chem. 2020, e26503. doi: https://doi.org/10.1002/qua.26503
Jasi?ski, R.; Jasi?ska, E.; Dresler, E. J. Mol. Model. 2016, 23, 1-9. doi: https://doi.org/10.1007/s00894-016-3185-8
Domingo, L. R.; Ríos-Gutiérrez, M.; Pérez, P. Org. Biomol. Chem. 2016, 14, 10427-10436. doi: https://doi.org/10.1039/C6OB01989G.
Domingo, L. R.; Aurell, M. J.; Pérez, P. Tetrahedron 2015, 71, 1050-1057. doi:https://doi.org/10.1016/j.tet.2014.12.094
Domingo, L. R.; Aurell, M. J.; Pérez, P. Tetrahedron 2014, 70, 4519-4525. doi: https://doi.org/10.1016/j.tet.2014.05.003.
Bimanand, A. Z.; Houk, K. N. Tetrahedron Lett. 1983, 24, 435-438. doi:https://doi.org/10.1016/S0040-4039(00)81429-X
Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H., Gaussian 16. Revision A 2016, 3.
Parr, R. G.; Weitao, Y., Density-Functional Theory of Atoms and Molecules. Oxford University Press: 1989.
Khabashesku, V. N.; Kudin, K. N.; Margrave, J. L. Russ. Chem. Bull. 2001, 50, 20-28. doi: https://doi.org/10.1023/A:1009508730904
Lemal, D. M. J. Org. Chem. 2017, 82, 13012-13019. doi: https://doi.org/10.1021/acs.joc.7b01911
Ditchfield, R.; Hehre, W. J.; Pople, J. A. 1971, 54 , 724-728. doi: https://doi.org/10.1063/1.1674902.
Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785-789. doi: https://doi.org/10.1103/PhysRevB.37.785.
Barone, V.; Cossi, M. J. Phys. Chem. A 1998, 102, 1995-2001. doi: https://doi.org/10.1021/jp9716997.
Fukui, K. J. Phys. Chem. 1970, 74, 4161-4163. doi: https://doi.org/10.1021/j100717a029
Domingo, L. R. RSC Adv. 2014, 4, 32415-32428. doi: https://doi.org/10.1039/C4RA04280H.
Pettersen, E. F.; Goddard, T. D.; Huang, C. C.; Couch, G. S.; Greenblatt, D. M.; Meng, E. C.; Ferrin, T. E. J. Comput. Chem. 2004, 25, 1605-1612. doi: https://doi.org/10.1002/jcc.20084
Parr, R. G.; Pearson, R. G. J. Am. Chem. Soc. 1983, 105, 7512-7516. doi: https://doi.org/10.1021/ja00364a005.
Parr, R. G.; Weitao, Y., Density-Functional Theory of Atoms and Molecules. Oxford University Press: 1994. DOI: https://doi.org/10.1093/oso/9780195092769.001.0001
Parr, R. G.; Szentpály, L. v.; Liu, S. J. Am. Chem. Soc. 1999, 121, 1922-1924. doi: https://doi.org/10.1021/ja983494x.
Kohn, W.; Sham, L. J. Phys. Rev. 1965, 140, A1133-A1138. doi: https://doi.org/10.1103/PhysRev.140.A1133.
Domingo, L. R.; Chamorro, E.; Pérez, P. J. Org. Chem. 2008, 73, 4615-4624. doi: https://doi.org/10.1021/jo800572a.
Becke, A. D.; Edgecombe, K. E. J. Chem. Phys. 1990, 92, 5397-5403. doi: https://doi.org/10.1063/1.458517.
Silvi, B.; Savin, A. Nature 1994, 371, 683-686. doi: https://doi.org/10.1038/371683a0.
Ríos-Gutiérrez, M.; Domingo, L. R. Eur. J. Org. Chem. 2019, 267-282. doi: https://doi.org/10.1002/ejoc.201800916.
Domingo, L. R.; Acharjee, N. ChemistrySelect 2018, 3, 8373-8380. doi: https://doi.org/10.1002/slct.201801528.
Acharjee, N.; Banerji, A. J. Chem. Sci. 2020, 132, 65. doi: https://doi.org/10.1007/s12039-020-01766-5.
Domingo, L. R.; Ríos-Gutiérrez, M.; Pérez, P. J. Org. Chem. 2018, 83, 2182-2197. doi: https://doi.org/10.1021/acs.joc.7b03093.
Domingo, L. R.; Ríos-Gutiérrez, M.; Pérez, P. Molecules 2016, 21, 748. doi: https://doi.org/10.3390/molecules21060748.
Geerlings, P.; De Proft, F.; Langenaeker, W. Chem. Rev. 2003, 103, 1793-1874. doi: https://doi.org/10.1021/cr990029p.
Domingo, L. R.; Aurell, M. J.; Pérez, P.; Contreras, R. Tetrahedron 2002, 58, 4417-4423. doi: https://doi.org/10.1016/S0040-4020(02)00410-6.
Domingo, L. R.; Pérez, P. Org. Biomol. Chem. 2011, 9, 7168-7175. doi: https://doi.org/10.1039/C1OB05856H.
Domingo, L. R.; Ríos-Gutiérrez, M.; Pérez, P. Molecules 2020, 25, 2535. doi: https://doi.org/10.3390/molecules25112535.
Krokidis, X.; Noury, S.; Silvi, B. J. Phys. Chem. A 1997, 101, 7277-7282. doi: https://doi.org/10.1021/jp9711508.

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









