Use of Chaya (Cnidoscolous chayamansa) Leaves for Nutritional Compounds Production for Human Consumption
DOI:
https://doi.org/10.29356/jmcs.v65i1.1433Keywords:
Cnidoscolus chayamansa, plant protein, RuBisCO, polyphenols, human nutritionAbstract
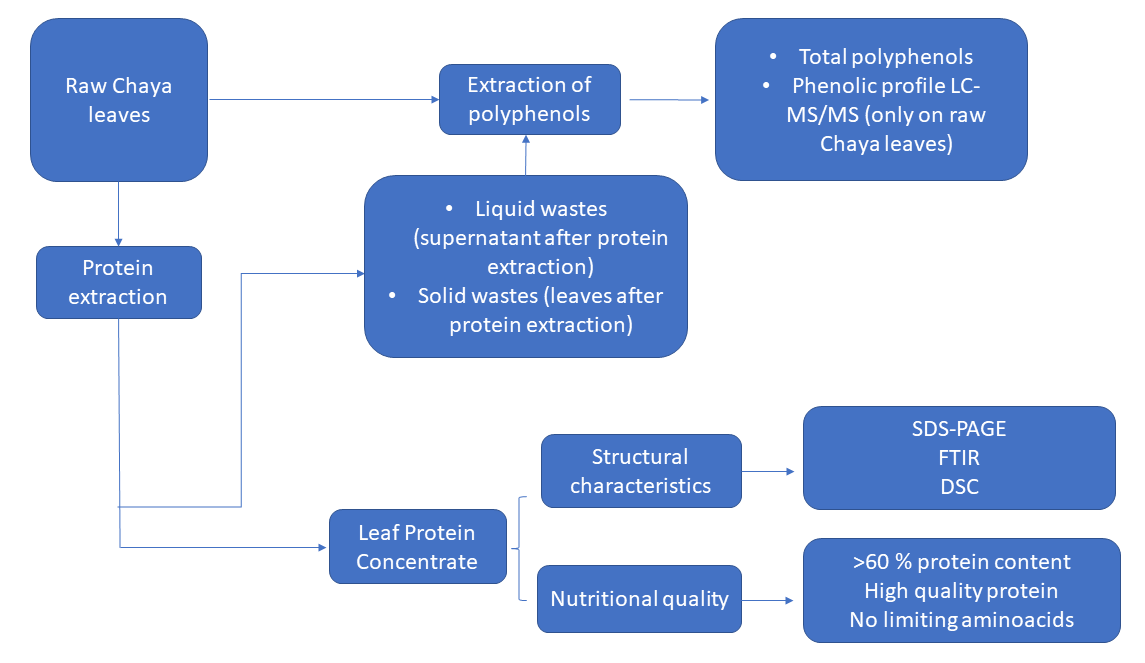
Abstract. Chaya (Cnidoscolus chayamansa) is an edible leafy vegetable consumed in the southeast region of México and Central America; it is mainly appreciated for its protein content and bioactive compounds, such as polyphenols. In this work, chaya leaves were nutritionally characterized and used for the production of protein concentrates. The nutritional quality (amino acids, protein efficiency, and bioavailability) and structure by SDS-PAGE, Fourier Transform Infrared Spectroscopy (FTIR), and Differential Scanning Calorimetry (DSC) of protein were analyzed. Additionally, the amount of some polyphenolic compounds were identified by LC-MS/MS. Electrophoretic bands from RuBisCO protein subunits at 10 kDa and 50 kDa were observed. FTIR identified the typical protein footprint of secondary structure and DSC measurements showed a denaturation temperature ~57 °C. Protein concentrates with a purity of 63.52 ± 0.69 % showed higher bioavailability than the control diet, and the essential amino acids met the FAO requirements without limiting amino acids. From the total polyphenol content, 14.8 % corresponded to anthocyanidins, 61.0 % hydroxycinnamic acids, 6.9 % hydroxybenzoic acids, and 17.2 % flavonols. Chaya leaves are a potential low-cost underutilized alternative source for the production of nutritional compounds for functional food product development for human nutrition.
Resumen. La chaya (Cnidoscolus chayamansa), es una hoja comestible que se consume en las regiones del sureste de México y Centroamérica, es apreciada principalmente por su contenido de proteínas y compuestos bioactivos, tales como los polifenoles. En este trabajo, las hojas de chaya fueron caracterizadas nutricionalmente y utilizadas para la producción de concentrados proteicos. Se estudió la calidad nutrimental de la proteína (aminoácidos, eficiencia proteica y biodisponibilidad) y algunas características estructurales mediante técnicas analíticas como SDS-gel electroforesis, espectroscopía de infrarrojo por la transformada de Fourier (FTIR) y calorimetría diferencial de barrido (DSC). Asimismo, se determinó la cantidad total de polifenoles y se identificó el perfil de polifenoles mediante LC-MS/MS. Se observaron las bandas electroforéticas correspondientes a las subunidades de la proteína RuBisCO a 10 kDa y 50 kDa. En el FTIR se identificó el espectro típico de la estructura secundaria de la proteína y DSC mostró una temperatura de desnaturalización ~57 °C. Los concentrados de proteína con pureza ~63.52 ± 0.69 % mostraron mayor biodisponibilidad que el control y la cantidad de aminoácidos esenciales cumplieron con los requerimientos de la FAO sin aminoácidos limitantes. Del total del contenido de polifenoles, 14.8 % correspondieron a las antocianidinas, 61.0 % a ácidos hidroxicinámicos, 6.9 % a ácidos hidroxibenzoicos y 17.2 % a flavonoles. Las hojas de chaya pueden ser una fuente potencial alternativa para la producción de compuestos nutrimentales para el desarrollo de productos alimentarios funcionales para la nutrición humana.
Downloads
References
Day, L. Trends Food Sci. Technol. 2013, 32, 25-42. DOI: https://doi.org/10.1016/j.tifs.2013.05.005
Boland, M. J.; Rae, A. N.; Vereijken, J. M.; Meuwissen, M. P.; Fischer, A. R.; van Boekel, M. A.; Rutherfurd, S. M.; Gruppen, H.; Moughan, P. J.; Hendriks, W. H. Trends Food Sci. Technol. 2013, 29, 62-73. DOI: https://doi.org/10.1016/j.tifs.2012.07.002
Edelman, M.; Colt, M. Frontiers in Chemistry. 2016, 4, 1-5. DOI: https://doi.org/10.3389/fchem.2016.00032
Kim, J. S.; Wilson, J. M.; Lee, S. R. J. Nutr. Biochem. 2010, 21, 1-13. DOI: https://doi.org/10.1016/j.jnutbio.2009.06.014
Semba, R. D.; Shardell, M.; Ashour, F. A. S.; Moaddel, R.; Trehan, I.; Maleta, K. M.; Ordiz, M. I.; Kraemer, K.; Khadeer, M. A.; Ferrucci, L.; Manary, M. J. EBioMedicine. 2016, 6, 246-252. DOI: https://doi.org/10.1016/j.ebiom.2016.02.030
Haque, M. A.; Timilsena, Y. P.; Adhikari, B., in: Reference Module in Food Science, Elsevier, 2016. DOI: https://doi.org/10.1016/B978-0-08-100596-5.03057-2.
Ross-Ibarra, J.; Molina-Cruz, A. Economic Botany. 2002, 56, 350-365
García-Rodríguez, R V.; Gutiérrez-Rebolledo, G. A.; Méndez-Bolaina, E.; Sánchez-Medina, A.; Maldonado-Saavedra, O.; Domínguez-Ortiz, M. Á.; Vázquez-Hernández, M.; Muñoz-Muñiz, O. D.; Cruz-Sánchez, J. S. Journal of Ethnopharmacology. 2014, 151, 937-943. DOI: https://doi.org/10.1016/j.jep.2013.12.004
Loarca?Piña, G.; Mendoza, S.; Ramos?Gómez, M.; Reynoso, R. Journal of Food Science. 2010, 75, H68-H72. DOI: https://doi.org/10.1111/j.1750-3841.2009.01505.x
Pérez-González, M. Z.; Gutiérrez-Rebolledo, G. A.; Yépez-Mulia, L.; Rojas-Tomé, I. S.; Luna-Herrera, J.; Jiménez-Arellanes, M. A. Biomedicine & Pharmacotherapy. 2017, 89, 89-97. DOI: https://doi.org/10.1016/j.biopha.2017.02.021
Pérez-González, M. Z.; Macías-Rubalcava, M. L.; Hernández-Ortega, S.; Siordia-Reyes, A. G.; Jiménez-Arellanes, M. A. Biomedicine & Pharmacotherapy. 2019, 117, 1-10. DOI: https://doi.org/10.1016/j.biopha.2019.109140.
Pérez-González, M. Z.; Siordia-Reyes, A. G.; Damián-Nava, P.; Hernández-Ortega, S.; Macías-Rubalcava, M. L.; Jiménez-Arellanes, M. A. Evidence-Based Complementary and Alternative Medicine. 2018, 2018, 1-12. DOI: https://doi.org/10.1155/2018/3896517
Anil, K.; Alzahal, O.; Singh, K. K.; McBride, B. W. Indian Journal of Animal Sciences. 2010, 80, 1008-1010.
Aguilar-Ramírez, J.; Santos-Ricalde, R.; Pech-Martínez, V.; Montes-Pérez, R. Revista Biomédica. 2000, 11, 17-24. DOI: https://doi.org/10.32776/revbiomed.v11i1.215
Colas, D.; Doumeng, C.; Pontalier, P. Y.; Rigal, L. Food and Bioproducts Processing. 2013, 91, 175-182. DOI: https://doi.org/10.1016/j.fbp.2013.01.002
Association of Official Analytical Chemists. Official Methods of Analysis. AOAC International, 16th Ed., Washington DC, 2005.
Olvera-Novoa, M. A.; Martinez Palacios, C. A.; Real de Leon, E. Nutrition of fish and crustaceans: a laboratory manual. Food and Agriculture Organization of The United Nations-FAO, Mexico City, 1994.
Alaiz, M.; Navarro, J. L.; Girón, J.; Vioque, E. J. Chromatog. A. 1992, 591, 181-186. DOI: https://doi.org/10.1016/0021-9673(92)80236-N
Food and Agriculture Organization of the United Nations. FAO Food and Nutrition Paper. 2011, 92, 1-62.
Alsmeyer, R. H.; Cunningham, A. E.; Happich, M. L. Food Technol. 1974, 28, 34-40.
Laemmli, U. K. Nature. 1970, 227, 680-685.
Khan, S. A.; Shinwari, Z. K.; Rabbani, M. A. Pak. J. Bota. 2013, 45, 871-876.
Zhao, X.; Chen, F.; Xue, W.; Lee, L. Food Hydrocolloids, 2008, 22, 568–575. DOI: https://doi.org/10.1016/j.foodhyd.2007.01.019
Martín, A. H.; Nieuwland, M.; de Jong, G. A. H. J. Agric. Food Chem. 2014, 62, 10783-10791. DOI: https://doi.org/10.1021/jf502905g
Sarmiento?Franco, L.; Sandoval?Castro, C. A.; McNab, J. M.; Quijano?Cervera, R.; Reyes?Ramirez, R. R. J. Sci. Food Agric. 2003, 83, 609-612. DOI: https://doi.org/10.1002/jsfa.1372
Alain Mune Mune, M.; Nyobe, E. C.; Bakwo Bassogog, C.; Minka, S. R. Cogent Food Agric. 2016, 2, 1-8. DOI: https://doi.org/10.1080/23311932.2016.1213618
Hanif, R.; Iqbal, Z.; Iqbal, M.; Hanif, S.; Rasheed, M. J. Agricultur. Biologic. Sci. 2006, 1, 18-22.
Bukhsh, E.; Salman, A. M.; Sheikh, S. A. Pak. J. Bot. 2007, 39, 1181-1187.
Pereira, C.; Dias, M. I.; Petropoulos, S. A.; Plexida, S.; Chrysargyris, A.; Tzortzakis, N.; Calhelha, R. C.; Ivanov, M.; Stojkovi?, D.; Stojkovi?, M.; Barros, L.; Ferreira, I. C. F. R. Molecules. 2019, 24, 1-28. DOI: https://doi.org/10.3390/molecules24244494
Traoré, K.; Parkouda, C.; Savadogo, A.; Ba/Hama, F.; Kamga, R.; Traoré, Y. Food Sci. Nut. 2017, 5, 1139-1144. DOI: 10.1002/fsn3.504
Adeyeye, E. I.; Omolayo, F.O. Agric. Biol. J. North Am. 2011, 2, 499-511. DOI: https://doi:10.5251/abjna.2011.2.3.499.511
Khan, L. H.; Varshney, V. K. J. Diet. Suppl. 2018, 15, 386-397. DOI: https://doi.org/10.1080/19390211.2017.1349232
Saha, J.; Deka, S. C. Int. J. Food Prop. 2017, 20, 1051-1061. DOI: https://doi.org/10.1080/10942912.2016.1199034
Hojilla?Evangelista, M. P.; Selling, G. W.; Hatfield, R.; Digman, M. J. Sci. Food Agric. 2017, 97, 882-888. DOI: https://doi.org/10.1002/jsfa.7810
Rawdkuen, S. Food and Applied Bioscience Journal. 2020, 8, 43-47.
Ranhotra, G. S.; Gelroth, J. A.; Leinen, S. D.; Vinas, M. A.; Lorenz, K. J. J. Food Compos. Anal. 1999, 11, 298-304. DOI: https://doi.org/10.1006/jfca.1998.0590
Famuwagun, A. A.; Alashi, A. M.; Gbadamosi, S. O.; Taiwo, K. A.; Oyedele, D. J.; Adebooye, O. C.; Aluko, R. E. Int. J. Food Prop. 2020, 23, 955-970. DOI: https://doi.org/10.1080/10942912.2020.1772285
Agbede, J. O.; Aletor, V.A. Int. J. Food Sci. Technol. 2004, 39, 253–261. doi:10.1111/j.1365-2621.2004.00779.x
Zengin, G.; Aktumsek, A.; Guler, G. O.; Cakmak, Y. S.; Girón-Calle, J.; Alaiz, M.; Vioque, J. Food Chem. 2012, 135, 1360-1364. DOI: https://doi.org/10.1016/j.foodchem.2012.05.084
Rutherfurd, S. M.; Moughan, P. J. British J. Nut. 2012, 108, S298-S305. DOI: https://doi.org/10.1017/S0007114512002528
Montoya-Martínez, C.; Nolasco-Soria, H.; Vega-Villasante, F.; Carrillo-Farnés, O.; Álvarez-González, A.; Civera-Cerecedo, R. Latin Am. J. Aquatic Res. 2018, 46, 495-501. DOI: http://dx.doi.org/10.3856/vol46-issue3-fulltext-1.
Di Stefano, E.; Agyei, D.; Njoku, E. N.; Udenigwe, C. C. J. Am. Oil Chemists’ Soc. 2018, 95, 1063–1074. DOI: https://doi.org/10.1002/aocs.12104
Lamsal, B. P.; Koegel, R. G.; Gunasekaran, S. LWT - Food Sci. Tech. 2007, 40, 1520–1526. DOI: https://doi.org/10.1016/j.lwt.2006.11.010
Andrade, J.; Pereira, C. G.; de Almeida Junior, J. C.; Viana, C. C. R.; de Oliveira Neves, L. N.; da Silva, P. H. F.; Valenzuela Bell, M. J.; dos Anjos, V. D. C. LWT. 2019, 99, 166-172. DOI: https://doi.org/10.1016/j.lwt.2018.09.079
Karthikeyan, S.; Vizhiselvi, K. Indian Journal of Pure and Applied Physics. 2017, 55, 525-531.
Kong, J.; Yu, S. Acta Biochimica et Biophysica Sinica. 2007, 39, 549-559. DOI: https://doi.org/10.1111/j.1745-7270.2007.00320.x
Sazonova, S.; Grube, M.; Shvirksts, K.; Galoburda, R.; Gramatina, I. Journal of Molecular Structure. 2019, 1186, 377-383. DOI: https://doi.org/10.1016/j.molstruc.2019.03.038
Ramos?Gomez, M.; Figueroa?Pérez, M. G.; Guzman?Maldonado, H.; Loarca?Piña, G.; Mendoza, S.; Quezada?Tristán, T.; Reynoso?Camacho, R. J. Food Biochem. 2017, 41, 1-9. DOI: https://doi.org/10.1111/jfbc.12281
Valenzuela Soto, R.; Morales Rubio, M. E.; Verde Star, M. J.; Oranday Cárdenas, A.; Preciado-Rangel, P.; Antonio González, J.; Esparza-Rivera, J. R. Revista Mexicana de Ciencias Agrícolas. 2015, 6, 815-825.
Kuti, J. O.; Konuru, H. B. J. Agric. Food Chem. 2004, 52, 117–121. DOI: https://doi.org/10.1021/jf030246y
Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









