Adsorptive Removal of 2,4-Dichlorophenol from Aqueous Solution by Using Used Black Tea Leaves
DOI:
https://doi.org/10.29356/jmcs.v65i2.1424Keywords:
Adsorption kinetics, 2,4-DCP, sodium chlorite, used black tea leaves, removalAbstract
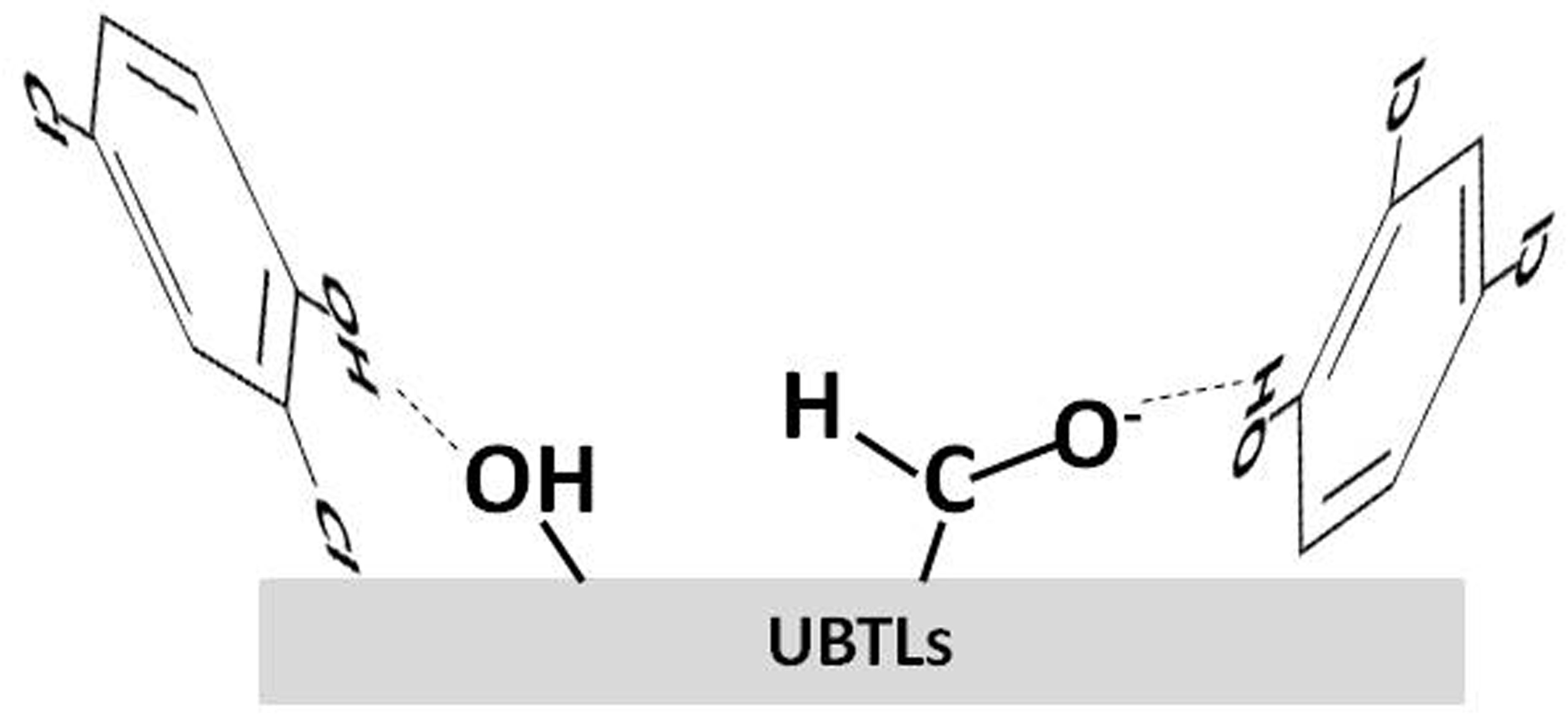
Abstract. Bioadsorbents are drawing the attention of the environmental scientists in removing organic pollutants from aqueous solution because of their availability and low cost. In this research, adsorptive removal of 2,4-dichlorophenol (2,4-DCP) onto used black tea leaves (UBTLs) as well as on sodium chlorite modified used black tea leaves (SCM-UBTLs) was investigated at different conditions. The value of pH was optimized at 2. Smaller particle size (50-100 mm) of both the adsorbents showed maximum removal of 2,4-DCP. However, SCM-UBTLs exhibited comparatively higher (54%) removal of DCP than unmodified used black tea leaves (UM-UBTLs) (40%) at similar conditions (pH, particle size and dose). Equilibrium attained within three hours for both the adsorbents of smaller particle size at pH 2. Adsorption follows the Ho’s pseudo-second-order kinetics rather than Lagergren pseudo-first- order for both the adsorbents. The experimental data was justified with the FTIR spectra of adsorbed and unadsorbed surfaces.
Resumen. Debido a su disponibilidad y bajo costo los bioadsorbentes están atrayendo la atención de los científicos ambientales para la eliminación de contaminantes orgánicos de soluciones acuosas. En esta investigación se analizó la eliminación, por adsorción, del 2,4-diclorofenol (2,4-DCP) en hojas de té negro usadas (UBTL), así como en hojas de té negro usadas tratadas con clorito de sodio (SCM-UBTL) bajo diferentes condiciones. El valor de pH se optimizó a 2. El tamaño de partícula más pequeño (50-100 µm), de ambos adsorbentes, mostró una eliminación máxima del 2,4-DCP. Sin embargo, los SCM-UBTL exhibieron una remoción de DCP comparativamente más alta (54%) que las hojas de té negro usadas sin modificar (UM-UBTL) (40%) en condiciones similares (pH, tamaño de partícula y dosis). El equilibrio se alcanzó en tres horas para ambos adsorbentes con el tamaño de partícula más pequeño a pH 2. La adsorción sigue la cinética de pseudo-segundo orden de Ho en lugar de la de pseudo-primer orden de Lagergren para ambos adsorbentes. Los datos experimentales se vieron apoyados con los espectros FTIR de superficies adsorbidas y no adsorbidas.
Downloads
References
Kintz, P.; Tracqui, A.; Mangin, P. Arch. Toxicol. 1992, 66, 298-299. DOI: https://doi.org/10.1007/BF02307178
Juang, R. S.; Wu, F.C.; Tseng, R. L. J. Chem. Eng. Data 1996, 41, 487-492. DOI: https://doi.org/10.1021/je950238g
Al-Asheh, S.; Banat, F.; Masad, A. Wat. Qual. Res. J. Canada 2005, 40, 211-221. DOI: https://doi.org/10.2166/wqrj.2005.024
Arellano-Cardenas, S.; Gallardo-Velazquez, T.; Osorio-Revilla, G.; Lopez-Cortezi, M. S.; Gomez-Perea, B. J. Mex. Chem. Soc. 2005, 49, 287-291.
Ghatbandhe, A. S.; Yenkie, M. K. J. Env.Sci. Eng. 2008, 50, 163-168.
Xuequan, Z.; Xiankai, W.; Huixiang, S.; Dahui, W. Tran. Tianj. Univ. 2009, 15, 408-414.
Shaarani, F. W.; Hameed, B. H. Chem. Eng. J. 2011, 169, 180-185. DOI: https://doi.org/10.1016/j.cej.2011.03.002
Hanzhong, J.; Chuanyi, W. Chem. Eng. J. 2012, 191, 202-209.
Guang-qian, W.; Xin-yuan, S.; Hui, H.; Xin, Z.; Jie, Y.; Qi-sheng, Z. Des. Wat. Treat. 2013, 51, 4603-4612.
Islam, T. S. A.; Begum, H. A.; Hossain, M. A.; Moniruzzaman, M. J. Bang. Aca. Sci. 2013, 37, 1-10. DOI: https://doi.org/10.3329/jbas.v37i1.15674
Li, W.; Jian, Z.; Ran, Z.; Chenglu, Z.; Cong, L.; Ye, Li. Des. 2011, 266, 175-181.
Jian-Wei, M.; Hui, W.; Fa-Yuan, W.; Zheng-Hong, H. Sep. Sci. Tech. 2010, 45, 2329-2336.
Yi, L.; Jintao, W.; Yian, Z.; Aiqin, W. Chem. Eng. J. 2012, 184, 248-255.
Malakootian, M.; Mansoorian, H. J.; Alizadeh, M.; Baghbanian, A. Sci. Ira. C. 2017, 24, 3041-3052.
Kusmierek, K.; Szala, M.; Swiatkowski, A. J. Tai. Ins. Chem. Eng. 2016, 63, 371-378. DOI: https://doi.org/10.1016/j.jtice.2016.03.036
Senthil, K. P.; Ramalingam, S.; Senthamarai, C.; Niranjanaa, M.; Vijayalakshmi, P.; Sivanesan, S. Des. 2010, 261, 52-60. DOI: https://doi.org/10.1016/j.desal.2010.05.032

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









