Synthesis, Characterization and Cytotoxicity Investigation of Chitosan-Amino Acid Derivatives Nanoparticles in Human Breast Cancer Cell Lines
Cytotoxicity Investigation of Chitosan-Amino Acid Derivatives Nanoparticles
DOI:
https://doi.org/10.29356/jmcs.v65i2.1265Keywords:
Chitosan-grafted-amino acids, nanoparticles, breast cancer cell lines, cytotoxicity, MTTAbstract
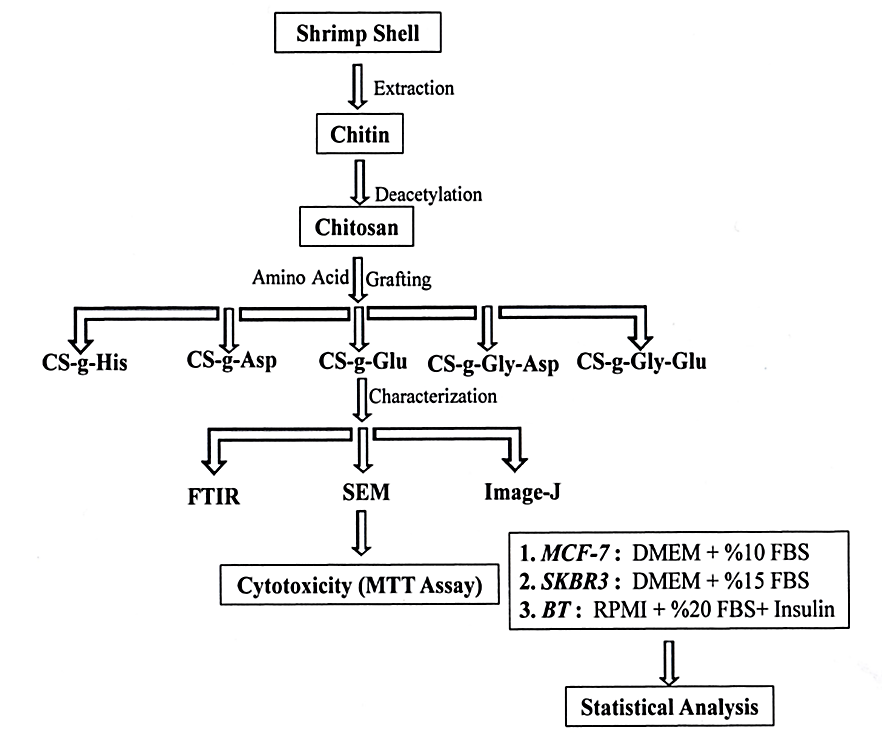
Abstract. The presence of reactive primary amines in the backbone structure of chitosan enables the derivatization with different functional groups and thereby improving and expanding its properties, such as solubility and mucoadhesiveness, for biomedical applications. In this work, chitosan was grafted with different sources of amino acids (Histidine, Aspartic acid, Glutamic acid, Glycine-Aspartic acid, and Glycine-Glutamic acid), Chitosan and its grafted amino acid derivatives were obtained in very good yield, and they were characterized by Fourier-Transform Infrared Spectroscopy (FTIR), and the resulted spectra confirmed the right structures of chitosan and its different synthesized derivatives. The chitosan and its amino acid derivatives were converted to nanoparticles in size by subjecting them to the sonication method. The Scanning Electron Microscope (SEM) was used to determine the shape and size of the prepared polymeric nanoparticles and the average nanoparticle size counted by the Image-J program. The micrographs revealed that the nanoparticles with spherical shapes and with different sizes were gained, but in general, they are less than 100nm in diameters. In vitro cytotoxicity of chitosan and chitosan derivatives prepared NPs were determined as MTT assay, against different three types of human breast cancer cell lines which are BT cell lines, MCF-7 cell lines, and SKBR3 cell lines. The cell proliferation of each type of breast cancer cell line has appeared to a highly significant decrease (p<0.001), with all types of tested NPs polymers in comparison with the positive control samples, through different periods of the experiment (24, 48, and 72 hours).
Resumen. La presencia de aminas primarias reactivas en la estructura del quitosano permite su funcionalización con diferentes grupos funcionales, mejorando y expandiendo sus propiedades, por ejemplo, solubilidad y mucoadhesividad, para aplicaciones biomédicas. En este trabajo se injertó quitosano con diferentes fuentes de aminoácidos (histidina, ácido aspártico, ácido glutámico, glicina-ácido aspártico y glicina-ácido glutámico). Los derivados de quitosano injertados con aminoácidos se obtuvieron con muy buen rendimiento. La caracterización por espectroscopía infrarroja (FTIR) confirmó la funcionalización del quitosano. Después de sonicación y una caracterización por microscopía electrónoica de barrido (SEM), se confirmó que el tamaño del quitosano y sus derivados con aminoácidos pueden clasificarse como nanopartículas. Las micrografías revelaron que las nanopartículas tienen formas esféricas y son de diferentes tamaños, pero en general, son menores a 100 nm de diámetro. La citotoxicidad in vitro de las nanopartículas de quitosano y derivados de quitosano se determinó como ensayos MTT frente a tres tipos diferentes de líneas celulares de cáncer de mama humano, a saber, líneas celulares BT, líneas celulares MCF-7 y líneas celulares SKBR3. La proliferación celular de cada tipo de línea celular de cáncer de mama mostró una disminución significativa (p <0.001), con todos los tipos de polímeros NP probados en comparación con las muestras de control positivo, a lo largo de diferentes períodos del experimento (24, 48, y 72 horas).
Downloads
References
Song, Z.; Li G.; Guan, F.; Liu, W. Polymers 2018, 10, 389-402. DOI: https://doi.org/10.3390/polym10040389
Ibitoye, E. B.; Lokman, I. H.; Hezmee, M. N. M.; Goh, Y. M.; Zuki, A. B. Z.; Jimoh, A. A. Biomed. Mater. 2018, 13, 1-13. DOI: https://iopscience.iop.org/article/10.1088/1748-605X/aa9dde DOI: https://doi.org/10.1088/1748-605X/aa9dde
Mutasher, S. H.; Salih, A. A.; Al-Lami, H. S. Der. Pharma. Chemica 2016, 8, 125-134. http://derpharmachemica.com/archive.html
Li, Z.; Yang, F.; Yang, R. Int. J. Biol. Macromol. 2015, 75, 378-387. DOI: https://doi.org/10.1016/j.ijbiomac.2015.01.056
Gu?oaia, A. Chitosan Nanoplexes for target siRNA deliver, Ph.D. thesis, the University of Tübingen, aus Gala?i, Rumänien, 2015. https://publikationen.uni-tuebingen.de
Raftery, R; O’Brien, R. F. J.; Cryan, S. Molecules 2013, 18, 5611- 5647. DOI: https://pubmed/ 10.3390/molecules18055611 DOI: https://doi.org/10.3390/molecules18055611
Jennings, J. A.; Bumgardner, J. D. Chitosan Based Biomaterials, Volume 2: Tissue Engineering and Therapeutics, 1st ed.; Elsevier Ltd.: United Kingdom, 2017, 1-10. https://www.elsevier.com/books/chitosan-based-biomaterials-volume-2/jennings/978-0-08-100228-5
Carballal, B.; Fernández, E. F.; Goycoolea, F. M. Polymers (Basel) 2018, 10, 444, 1-51. DOI: https://ncbi/10.3390/polym10040444 DOI: https://doi.org/10.3390/polym10040444
Zhang, P.; Wagner, E., Top Curr. Chem. (Z) 2017, 375, 1-39. DOI: https://pubmed.ncbi/ 10.1007/s41061-017-0112-0 DOI: https://doi.org/10.1007/s41061-017-0112-0
Gad, A.; Kydd, J.; Piel, B.; Rai1, P. Int. J. Nanomed. Nanosurg. 2016, 2, 1-12. DOI: https://ncbi/10.16966/2470-3206.116 DOI: https://doi.org/10.16966/2470-3206.e103
Prabhu, R. H.; Patravale, V. B.; Joshi, M. D. Int. J. Nanomed. 2015, 10, 1001-1018. DOI: https://ncbi/10.2147/IJN.S56932
Chen, G.; Gong, R. Saudi Pharm. J. 2016, 24, 250-253. DOI: https://ncbi/10.1016/j.jsps.2016.04.008 DOI: https://doi.org/10.1016/j.jsps.2016.04.008
Lounis, F.M.; Chamieh, J.; Leclercq, L.; Gonzalez, P.; Rossi, J.; Cottet, H. Polymers 2018, 10, 45, 1-16. DOI: https://mdpi/10.3390/polym10010045 DOI: https://doi.org/10.3390/polym10010045
Hong, C. A.; Sonm, H. Y.; Nam, Y.S. Nature Sci. Rep. 2018, 8, 7738, 1-7. DOI: https://nature/10.1038/s41598-018-25655-7
Perni, S.; Prokopovich, P. Nanom.: Nanotech. Bio. Med. 2017, 13, 539-548. DOI: https://doi.org/10.1016/j.nano.2016.10.001
Gabano, E.; Quental, L.; Perin, E.; Silva, F.; Raposinho, P.; Paulo, A.; Ravera, M. Inorganics 2018, 6, 1-13. DOI: https://doi.org/10.3390/inorganics6010004
Alshraim, M. O.; Sangi, S.; Harisa, G. I.; Alomrani, A. H.; Yusuf O.; Badran, M. M. Molecules 2019, 24, 1-14.DOI: https://pubmed.ncbi/10.3390/molecules24020250 DOI: https://doi.org/10.3390/molecules24020250
Hajipour, A. R.; Hosseinia, S. M.; Jajarmi, S., New J. Chem. 2017, 41, 7447-7452. DOI: https://doi.org/10.1039/C7NJ00595D
Galhoum, A. A.; Hassanc, K. M.; Desouky, O. A.; Masoud, A.; Akashid, M. T.; Sakaid Y.; Guibalb, E. React. Funct. Polym. 2017, 113, 13-22. DOI: https://doi.org/10.1016/j.reactfunctpolym.2017.02.001
Rani, M.; Agarwal, A.; Negi, Y. S. J. Biomat. Nanobiotech. 2011, 2, 71-84. DOI: http://SciRP.org/10.4236/jbnb.2011.21010 DOI: https://doi.org/10.4236/jbnb.2011.21010
Rani, M.; Agarwal, A.; Maharana, T; Negi, Y. S. Afr. J. Pharm. Pharmacol. 2010, 4, 35-54. URL: https://academicjournals.org/journal/AJPP/article-full-text-pdf/A86C79232822
Silva, V. J. D. Preparation and characterization of chitosan nanoparticles for gene delivery, M.Sc. thesis, University of Lisbon, Portugal, November 2013, 31-32. https://fenix.tecnico.ulisbon.pt
Mosmann, T. J Immunol Methods 1983, 65, 55-63. DOI: https://doi.org/10.1016/0022-1759(83)90303-4
El-Ghaffar, M. A. A.; Akl, M. A.; Kamel, A .M.; Hashem, M. S., Egypt J. Chem. 2017, 60, 507-518. DOI: https://ejchem.journals.ekb.eg/10.21608/ejchem.2017.745.1021
Zaboon, M. H. Synthesis and Characterization of some New Polymeric Chitosan Nanoparticles Derivatives as Antitumor for Human Breast Cancer Cell Lines, Ph.D. thesis, University of Basrah, Iraq, Janaury 2019, 44-63.
Ferrari, M.; Fornasiero, M. C.; Isetta, A. M. J. Immun. Meth. 1990, 131, 165-172. DOI: https://pubmed.ncbi.10.1016/0022-1759(90)90187-z DOI: https://doi.org/10.1016/0022-1759(90)90187-Z
Casettari, L.; Vllasaliu, D.; Lam, J. K.; Soliman, M; Illum, L. Biomaterials 2012, 33, 7565–7583. DOI: https://doi.org/10.1016/j.biomaterials.2012.06.104
Al-Lami, H. S.; Zaboon, M. H.; Saleh, A. A. IOP Conf. Ser.: Mater. Sci. Eng. 2020, 871, 1-15. DOI: https://iopscience.iop.org/article/10.1088/1757-899X/871/1/012024 DOI: https://doi.org/10.1088/1757-899X/871/1/012024
Wernersson, E.; Heyda, J.; Kubícková, A.; Krízek, T.; Coufal, P.; Jungwirth, P. J. Phys. Chem. B. 2010, 114:11934–11941. DOI: https://doi.org/10.1021/jp1054342
Raja, M. A.; Zeenat, S. Arif, M.; Liu, C. Int. J. Nanomed. 2016, 11, 4397–4412.DOI https://doi.org/10.2147/IJN.S106116
Layek, B.; Singh, J. Biomacromolecules 2013, 14, 485-494. DOI: https://doi.org/10.1021/bm301720g

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









