Allyl Alcohol Production by Conversion of Glycerol Over Supported Iron and Nickel Bimetallic Oxides
DOI:
https://doi.org/10.29356/jmcs.v64i4.1246Keywords:
Iron oxides, glycerol, magnetite, hematite, allyl alcohol, FexOY-NiOAbstract
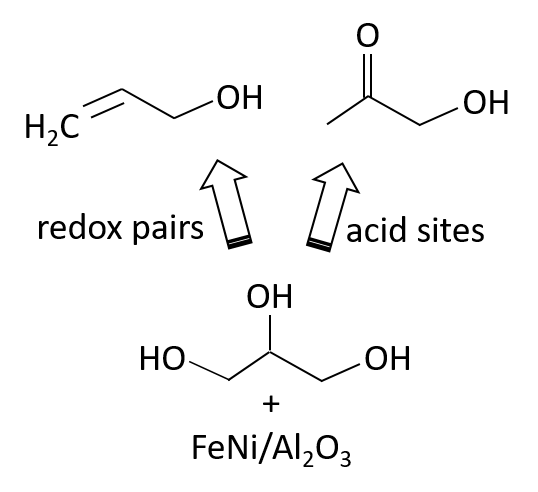
Abstract. The study of catalysts based on iron, nickel, and iron-nickel oxides (Fe, Ni and FeNi) supported on a commercial mesoporous alumina is presented. The metal oxides were synthesized by the incipient wetness impregnation method. The samples were characterized by XRD, TPR-H2, Raman spectroscopy, XPS and N2 adsorption. The catalytic activity was evaluated in the dehydration-dehydrogenation reaction of glycerol in the gas phase. In the iron oxide and nickel oxide catalysts, the result of TPR-H2 showed that the presence of nickel influences the reduction of iron species. In the reaction, the catalysts showed similar conversion. However, the selectivity of the bimetallic oxide catalysts is modified, and the production of allyl alcohol increases noticeably forming a greater amount allyl alcohol than that observed in the monometallic oxides, in which a greater amount of hydroxyacetone is formed. The results indicate that the redox pairs Fe+3/Fe+2 influence the selectivity of these oxides. A decrease in the ratio Fe+3/Fe+2 species favored the formation of allyl alcohol.
Resumen. Se presenta el estudio de catalizadores basados en óxidos de hierro y níquel (Fe, Ni y FeNi) soportados en una alúmina mesoporosa comercial. Los óxidos metálicos se sintetizaron por el método de impregnación de humedad incipiente. Las muestras se caracterizaron por XRD, TPR-H2, espectroscopía Raman, XPS y adsorción de N2. La actividad catalítica se evaluó en la reacción de deshidratación-deshidrogenación de glicerol en la fase gaseosa. En los catalizadores de óxido de hierro y óxido de níquel, el resultado de TPR-H2 mostró que la presencia de níquel influye en la reducción de las especies de hierro. En la reacción, los catalizadores mostraron una conversión similar. Sin embargo, la selectividad en los catalizadores de óxidos bimetálicos se modifica formando una mayor cantidad de alcohol alílico que la observada en los óxidos monometálicos, donde se forma una mayor cantidad de hidroxiacetona. Los resultados obtenidos indican que los pares redox Fe+2/Fe+3 influyen en el comportamiento catalítico de estos óxidos, en la selectividad. Una disminución de la relación Fe+3/Fe+2 favorece la formación de alcohol alílico.
Downloads
References
M. Feyzi y A.A. Mirzaei. Petroleum Chemistry, 2012, 5, 362-371. DOI: /10.113/SO96554411205002.
J.A.H. Dreyer, H.K. Grossmann, J. Chen, T. Greb, B.B. Gong, P.H.L. Sit, L. Madler, W.Y. Teoh. J. of Catalysis, 2015, 329, 248-261. DOI:/10.1016/j.jcat2015.5.003.
X. Li, W. Liv, J. Ma, Y. Wen, Z. Wu. Appl.Catal B, 2015, 179, 239-248. DOI: /10 1016/apcatb.2015.05.034.
T. Margossian, K. Larnier, F. Kumeich, C. Muller, C. Coperet. ACS Catalysis, 2017, 7 (10), 6942-6948. DOI: /10.1021/acscatal.7b02091.
M. Lubbe, A M. Gigler, R. W. Stark and W. Moritz. Surface Science, 2010, 604, 679-685. DOI: /10.1016/j.susc.2010.01.015.
Y. Liu, H. Tuysuz, Ch. J. Jia, M. Schwickardi, R. Rinaldi, A. H. Lu, W. Schmidt and F. Shut. Chem. Comm., 2010, 46, 1238. DOI: /10.139/b921648k.
F.J. García-Delgado, M. Viniegra, G. Córdoba y N. Martín. Actas XXV CICAT, 2016, P447, Montevideo, Uruguay.
E. Hong, S. Bang, J.H. Cho, K.D. Jung and C.H. Shin. Appl. Catal. A., 2017, 542, 146-153. DOI: /10.1016/j.apcata.2017.05.003.
A.S. De Oliveira, S.T. Vasconcelos, J. R. De Sousa, J.M. Filho, A. E. Oliveira. Chem. Eng. J., 2011, 168, 765. DOI: /10.1016/j.cej.2020.09.029.
R.C.R. Santos, D.M.V. Braga, Pinheiro A.N., Freire V.N., Longhinotti and Valentini A. Catal. Sci. &Tecnology, 2016, 6, 4986-5002. DOI: /10.1039/c6cy0096g.
M. Martínez-Rico, J. Aguilar-Pliego, J. Pérez-Pariente, C. Márquez, M. Viniegra and N. Martin. RMIQ, 2018, 17 (2), 523-532. DOI: /10.24275/uam/izt/dcbi/ revmexingquimica/ 2018v17n2/Martinez/.
T.L. Ma, Z. Yun, W. Xu, L. G. Chen, L. Li, J. F. Ding, R. Shan. Chem. Eng. J., 2016, 294, 343-352.
T. Yoshikawa, T. Tago, A. Nakamura; A. Konaka, M. Mukaida and T. Masuda. Res. Chem. Intermed., 2011, 37, 1247-1256. DOI: /10.1007/s1164-011-0391-y.
A. Ulgen and W. F. Hoelderich. Applied Catalysis A: Gen., 2011, 400: 34-38. DOI: 10.1016 /j.apcata.2011.04.005.
R. Almeida, M. Filipa Ribeiro, A. Fernandes and J. P. Lourenco. Catalysis Communication, 127, 2019, 20-24. DOI: 10.1016/j.catcom.2019.04.015.
G. Sánchez, B. Z. Dlugogorski, E. M. Kennedy and M. Stockenhuber. Applied Catalysis: A, 2016, 519, 130-142. DOI: /10.1016/apcata.2015.09.039.
C. A. Emeis. J. of Catalysis, 1993, 141, 347-354. DOI: 0021.9517.
D.L.A. de Faira, S. Venancio Silva and M.T. Oliveira. J. of Raman Spectroscopy, 1997, 28, 873-878. DOI: CCC 0377-0486/97/110873-06.
C. Gerónimo-López, J. Vázquez-Arenas, M. Picquart and I. González. Electrochimica Acta, 2014, 136, 146-156. DOI: /10.1016/j.electacta.2014.05.069.
D. Wu, Y. Zhang, M. Wen, H. Fang and Q. Wu. Inorganic Chemistry, 2017, 5152-5157. DOI: /10.1021/acs.inorg.chem.7b00304.
I. Martinuzzi, Y. Azizi, J.F. Davaux, S. Tretjak, O. Zahraa, J.P. Leclerk. Chem. Eng. Sci., 2014, 116, 118-127. DOI: /10.1016/j.ces.2014.04.030.
Y. M. Jin, A. K. Datye. J. of Catalysis, 2000, 196, 8-17. DOI: /10.1006/jcat.2000.3024.
D. Tian, Z. Liu, D. Li, H. Shi, W. Pan, Y. Chang. Fuel, 2013, 104, 224-229. DOI: /10.1016/j.fuel.2012.08.033.
D. Topaloglu-Yazici and C. Bilgic. Surf. Interface Anal., 2010, 42, 959-962. DOI: /10.1002/sia.3474.
H. Lang, X. Xiao, S. Yuan, B. Xhang, G. Zhou and Y. Yiang. Catal. Letters, 2017, 147, 2187-2199. DOI:/10.107/s10562-017-2124-3
A. Kostyniuk, D. Bajec, P. Djinovic and B. Likozar. Chem. Eng. Journal, 2020, 397, 125430. DOI:/10.1016/j.cej.2020.125430.
G. Sánchez, J. Friggieri, C. Keast, M. Drewery, B.Z. Dlugogorski, E. Kennedy and M. Stockenhuber. Appl. Catal. B, 2014, 152-153, 117-128. DOI:/10.1016/ j.apcatb.2014.01.019.
Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









