Adsorption of Lead (Ii) from Aqueous Solution Using Adsorbents Obtained from Nanche Stone (Byrsonima Crassifolia)
DOI:
https://doi.org/10.29356/jmcs.v64i4.1201Keywords:
Adsorption, nanche stone, Pb(II)Abstract
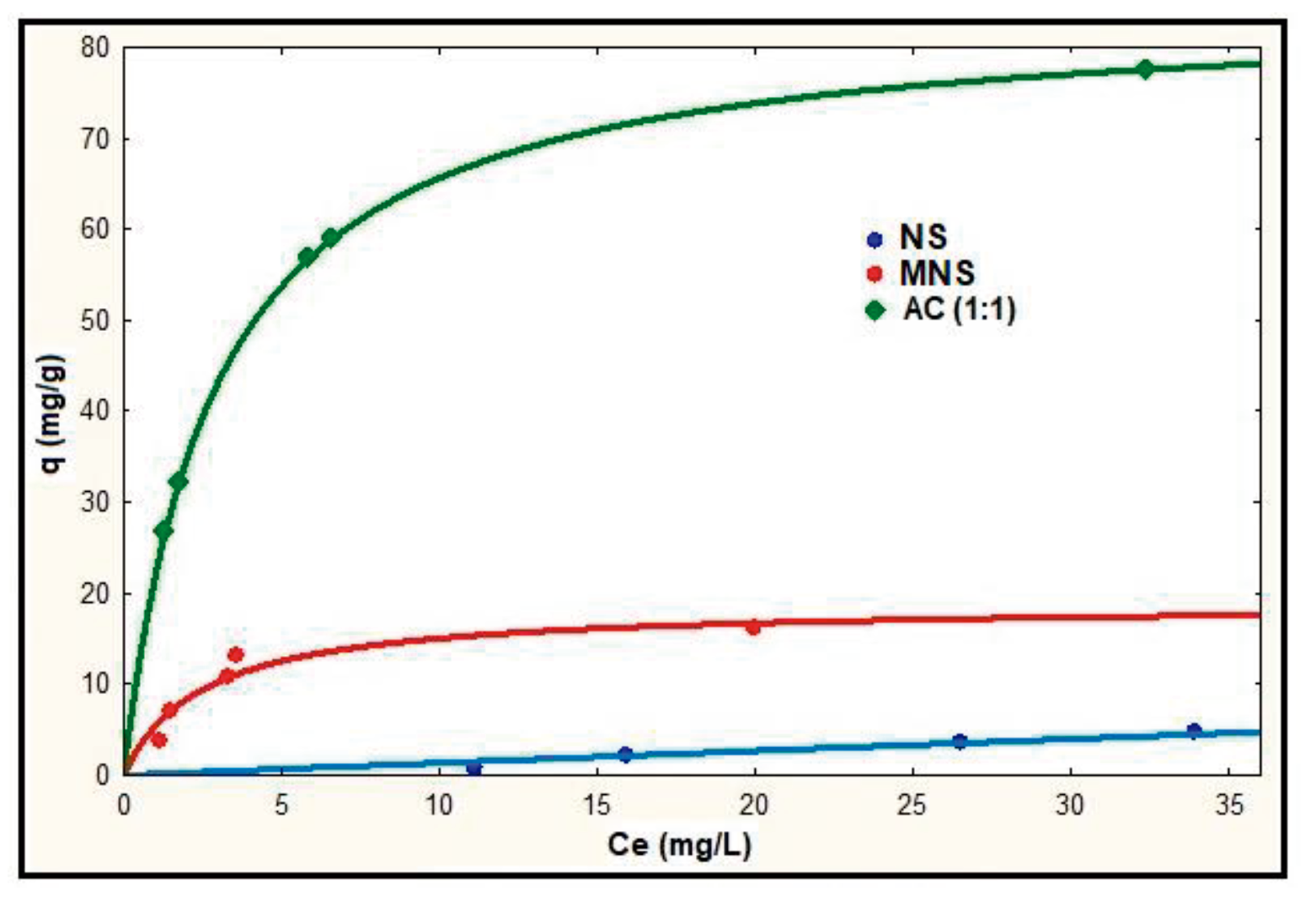
Abstract. The removal capacity of Pb(II) present in aqueous solution was evaluated, using as a sorbent the nanche stone (Byrsonima crassifolia) naturally (NS), modified with citric acid (MNS) and as activated carbon (AC). The point of zero charge (pHPZC) and the active sites (using the Boehm method and FTIR spectroscopy) were determined. The pHPZC of NS, MNS and AC were in an acid range. The concentration of active sites of NS, MNS and AC were 0.1037, 0.1123 y 0.1404 mol/g, respectively. The infrared spectra (FTIR) detected the formation of acid functional sites associated with the phenol group, carboxylic acids and lactones. The adsorption capacity of lead ions using NS, MNS and AC increases with the increment of the pH of the solution from 3 to 5; nevertheless, at pH 5, precipitation of lead ions is observed. Due to the above, the evaluation of the three materials was carried out at pH 4. Comparing the maximum capacity of adsorption of Pb(II) on NS with respect to MNS and AC at pH 4, it was increased in 2.2 and 10 times, respectively. The chemical modification applied to precursor, as well as its formation to AC, improved their adsorption capacity due to a greater generation of acid sites. The experimental data were represented with the models of Langmuir, Freundlich y Prausnitz-Radke and the parameter values of these isotherms were estimated using a least-squares method which utlilizes an optimization algorithm.
Resumen. Se evaluó la capacidad de remoción de Pb(II) presente en solución acuosa, utilizando como adsorbente el hueso de nanche (Byrsonima crassifolia) de forma natural (HN), modificado con ácido cítrico (HNM) y como carbón activado CA. Se determinaron el punto de carga cero (pHPZC) y los sitios activos (utilizando el método Boehm y espectroscopía FTIR). El pHPZC del HN, HNM y CA estuvo en un rango ácido. La concentración de sitios ácidos del HN, HNM y CA fueron de 0.1037, 0.1123 y 0.1404 mol/g respectivamente. Los espectros infrarrojos (FTIR), detectaron la formación de sitios funcionales ácidos asociados al grupo fenol, ácidos carboxílicos y lactonas. La capacidad de adsorción del ion plomo con el HN, HNM y CA, aumentó al incrementarse el pH de la solución de 3 a 5; sin embargo, a pH 5, se observó precipitación de iones plomo. Debido a lo anterior, la evaluación de los tres materiales se realizó a pH 4. Comparando la máxima capacidad de adsorción de Pb(II) en el HN con respecto al HNM y al CA a pH 4, se incrementó en 2.2 y 10 veces, respectivamente. La modificación química aplicada al precursor, así como su formación a CA, amplió su poder de adsorción al desarrollarse una mayor cantidad de sitios activos ácidos. Los datos experimentales se representaron con los modelos de Langmuir, Freundlich y Prausnitz-Radke, y los valores de los parámetros de estas isotermas fueron estimados usando un método de mínimos cuadrados que utiliza un algoritmo de optimización.
Downloads
References
Emenike, P.; Omole, D.; Ngene, B.; Tenebe, I. Global J. Environ. Sci. Manage. 2016, 2, 411-442. https://dx.doi.org/10.22034/gjesm.2016.02.04.010
Sulyman, M.; Namiesnik, J.; Gierak, A. Pol. J. Environ. Stud. 2017, 26, 479-510. https://dx.doi.org/10.15244/pjoes/66769 DOI: https://doi.org/10.15244/pjoes/66769
Tejada, C.; Bonilla, H.; Pino, J.; Villabona, A.; Ortega, R. Rev. Mex. Ing. Quím. 2020, 19, 1413-1423. https://doi.org/10.24275/rmiq/IA1101
Zhu, B.; Fan, T.; Zhang, D. J. Haz. Mat. 2008, 153, 300-328. https://doi.org/10.1016/j.jhazmat.2007.08.050
Ozdemir, I.; Sahin, M.; Orhan, R.; Erdem, M. Fuel Process. Technol. 2014, 125, 200-206. http://dx.doi.org/10.1016/j.fuproc.2014.04.002 DOI: https://doi.org/10.1016/j.fuproc.2014.04.002
Obayomi, K. S.; Bello, J. O.; Nnoruka, J. S.; Adediran A. A.; Olajide, P.O. Cogent Eng. 2019, 6, 1-17. https://doi.org/10.1080/23311916.2019.1687274
Malekian, F.; Ghafourian, H.; Zare, K.; Sharif, A. A.; Zamani, Y. J. Mex. Chem. Soc. 2019, 63, 120-129. http://dx.doi.org/10.29356/jmcs.v63i2.666 DOI: https://doi.org/10.29356/jmcs.v63i2.666
Medina, R.; Salazar, S.; Gómez, J. HortScience 2004, 39, 1070-1073. https://doi.org/10.21273/HORTSCI.39.5.1070
Bayuelo, J.; Lozano, J.; Ochoa, I. Rev. Fitotec. Mex. 2006, 29, 31-36. DOI: https://doi.org/10.35196/rfm.2006.Especial_2.31
Caballero, A.; Vela, G.; Pérez, J.; Escobar, R.; Ballinas, J. Etnobiología 2012, 10, 50-55.
Guzmán, A.; Cruz, E.; Miranda, C. Rev. Mex. Cien. For. 2013, 4, 82-89. https://doi.org/10.29298/rmcf.v4i20.372
Medina, R.; Ibarra, M. E.; González, J. G.; Salazar, S. Rev. Venez. Cienc. Tecnol. Aliment. 2017, 8, 45- 56. https://sites.google.com/site/1rvcta
Leyva, R.; Bernal, L. A.; Acosta, I. Sep. Purif. Technol. 2005, 45, 41-49. https://doi.org/10.1016/j.seppur.2005.02.005
Monroy, J.; Mendoza, D. I.; Bonilla, A.; Pérez, M. A. Int. J. Environ. Sci. Technol. 2015, 12, 2867–2880. https://doi.org/10.1007/s13762-014-0685-x]
Goertzen, S.; Thériault, K.; Oickle, A.; Tarasuk, A.; Andreas H. Carbon 2010, 48, 1252-1261. https://doi.org/10.1016/j.carbon.2009.11.050
Prado, M. A.; Arruda, S. M.; Ulson, A. A. J. Clean. Prod. 2014, 65, 342-349. http://dx.doi.org/10.1016/j.jclepro.2013.08.020 DOI: https://doi.org/10.1016/j.jclepro.2013.08.020
Chen, R.; Li, L.; Liu, Z.; Lu, M.; Wang, C.; Li, H.; Ma, W.; Wang, S. J. Air Waste Manage. Assoc. 2017, 67, 713-724. http://dx.doi.org/10.1080/10962247.2017.1280560 DOI: https://doi.org/10.1080/10962247.2017.1280560
Hock, P. E.; Ahmad, M. A. Acta Chimica Slovaca 2018, 11, 99-106. https://doi.org/10.2478/acs-2018-0015
Heidarinejad, Z.; Hadi, M.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Environ. Chem. Lett. 2020, 18, 393-415. https://doi.org/10.1007/s10311-019-00955-0
Giraldo, L.; Moreno, J. C. Braz. J. Chem. Eng. 2008, 25, 143-151. https://doi.org/10.1590/S0104-66322008000100015
Wang, G.; Zhang, S.; Yao, P.; Chen, Y.; Xu, X.; Li, T., Gong, G. Arab. J. Chem. 2018, 11, 99-110. http://dx.doi.org/10.1016/j.arabjc.2015.06.0117 DOI: https://doi.org/10.1016/j.arabjc.2015.06.011
Tasar, S.; Özer, A. Pol. J. Environ. Stud. 2020, 29, 293-305. https://doi.org/10.15244/pjoes/103027
Singh, J.; Ali, A.; Prakash, V. Int. J. Environ. Sci. Technol. 2014, 11, 1759-1770. https://doi.org/10.1007/s13762-013-0326-9
Shi, B.; Zuo, W.; Zhang, J.; Tong, H.; Zhao, J. J. Environ. Qual. 2016, 45, 984-992. https://doi.org/10.2134/jeq2014.12.0533
Nuithitikul, K.; Phromrak, R.; Saengngoen, W. Sci. Rep. 2020, 10, 1-14. https://doi.org/10.1038/s41598-020-60161-9
Bedia, J.; Peñas, M.; Gómez, A.; Rodríguez, J. J. Carbon Res. 2020, 6, 1-25. https://doi.org/10.3390/c6020021
Subha, R.; Namasivayam, C. Indian J. Chem. Technol. 2009, 16, 471-479. http://nopr.niscair.res.in/handle/123456789/6711
Acharya, J.; Sahu, J.; Mohanty, C.; Meikap, B. Chem. Eng. J. 2009, 149, 249-262. https://doi.org/10.1016/j.cej.2008.10.029
Angin, D. Fuel 2014, 115, 804-811. http://dx.doi.org/10.1016/j.fuel.2013.04.060 DOI: https://doi.org/10.1016/j.fuel.2013.04.060
Obike, A. I.; Igwe, J. C.; Emeruwa C. N.; Uwakwe, K. J. J. Appl. Sci. Environ. Manage. 2018, 22, 182-190. https://doi.org/10.4314/jasem.v22i2.5
Siripatana, C.; Khuenpetch, A.; Phromrak, R.; Saengngoen, W.; Nuithitikul, K. J. Eng. Applied Sci. 2017, 12, 1819-1824.
Celebi, h.; Gök, O. Int. J. Environ Res. 2017, 11, 83-90. https://doi.org/10.1007/s41742-017-0009-3
Saka, C.; Sahin, Ö.; Masuk, M. Int. J. Environ. Sci. Technol. 2012, 9, 379-394. https://doi.org/10.1007/s13762-012-0041-y
Martín, M. A.; Rodríguez, I. l.; Blázquez, G.; Calero, M. Desal. 2011, 278, 132-140. https://doi.org/10.1016/j.desal.2011.05.016
Bulut, Y.; Tez, Z. J. Haz. Mat. 2007, 149, 35-41. https://doi.org/10.1016/j.jhazmat.2007.03.044
Wolfová, R.; Pertile, E.; Fecko P. J. Environ. Chem. Ecotoxicol. 2013, 5, 159-167.DOI: 10.5897/JECE09.025
Singha, B.; Kumar, S. Environ. Sci. and Pollut. Res. 2012, 19, 2212-2226. https://doi.org/10.1007/s11356-011-0725-8
Li, Q.; Zhai, J.; Zhang, W.; Wang, M.; Zhou J. J. Haz. Mat. 2007, 141, 163–167. https://doi.org/10.1016/j.jhazmat.2006.06.109
Saleem, J.; Bin, U.; Hijab, M.; Mackey, H.; McKay, G. Biomass Conv. Bioref. 2019, 9, 775–802. https://doi.org/10.1007/s13399-019-00473-7
Jahangard, A.; Sohrabi, M.; Beigmohammadi, Z. Iran. J. Toxicol. 2016, 10, 23-29.https://doi.org/10.29252/arakmu.10.6.23
Bouchelkia, N.; Mouni, L.; Belkhiri, L.; Bouzaza, A.; Bollinger, J.; Madani, K. Separ. Sci.Technol. 2016, 51, 1645-1653. https://doi.org/10.1080/01496395.2016.1178289
Mouni, L.; Belkhiri, L., Zouggaghe, F., Tafer M. Desal. Water Treat. 2014, 52, 6412-6419. https://doi.org/10.1080/19443994.2013.812992
Alslaibi, T.; Abustan, I.; Ahmad, M.; Abu, A. Desal. Water Treat. 2014, 52, 7887-7897. https://doi.org/10.1080/19443994.2013.833875
Chaouch, N.; Ouahrani, M.; Laouini, S. Orient. J. Chem. 2014, 30, 1317-1322. http://dx.doi.org/10.13005/ojc/300349 DOI: https://doi.org/10.13005/ojc/300349
Ahmed, S.; Mohammad, S. Mycopath 2014, 12, 87-93.
Mohammadi, S.; Karimi, M.; Afzali, D.; Mansouri, F. Desal. 2010, 262, 86-93. https://doi.org/10.1016/j.desal.2010.05.048
Tangjuank, S.; Insuk, N.; Tontrakoon, J.; Udeye, V. Int. J. Chem. Mol. Nucl. Mat. Metall. Eng. 2009, 3, 221-227. https://doi.org/10.5281/zenodo.1074763
Tao, X.; Xiaoqin, L. Chin. J. Chem. Eng. 2008, 16, 401-406. https://doi.org/10.1016/S1004-9541(08)60096-8
Imamoglu, M.; Tekir, O. Desal. 2008, 228, 108-113. https://doi.org/10.1016/j.desal.2007.08.011

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









