Thermodynamics Properties of 1,1-Carbonyldiimidazole (CDI) and 4-Imidazole Acrylic Acid, Obtained by DSC and Combustion Calorimetry
DOI:
https://doi.org/10.29356/jmcs.v64i4.1193Keywords:
Imidazole, enthalpy of formation, melting poing, calorimetry, CDIAbstract
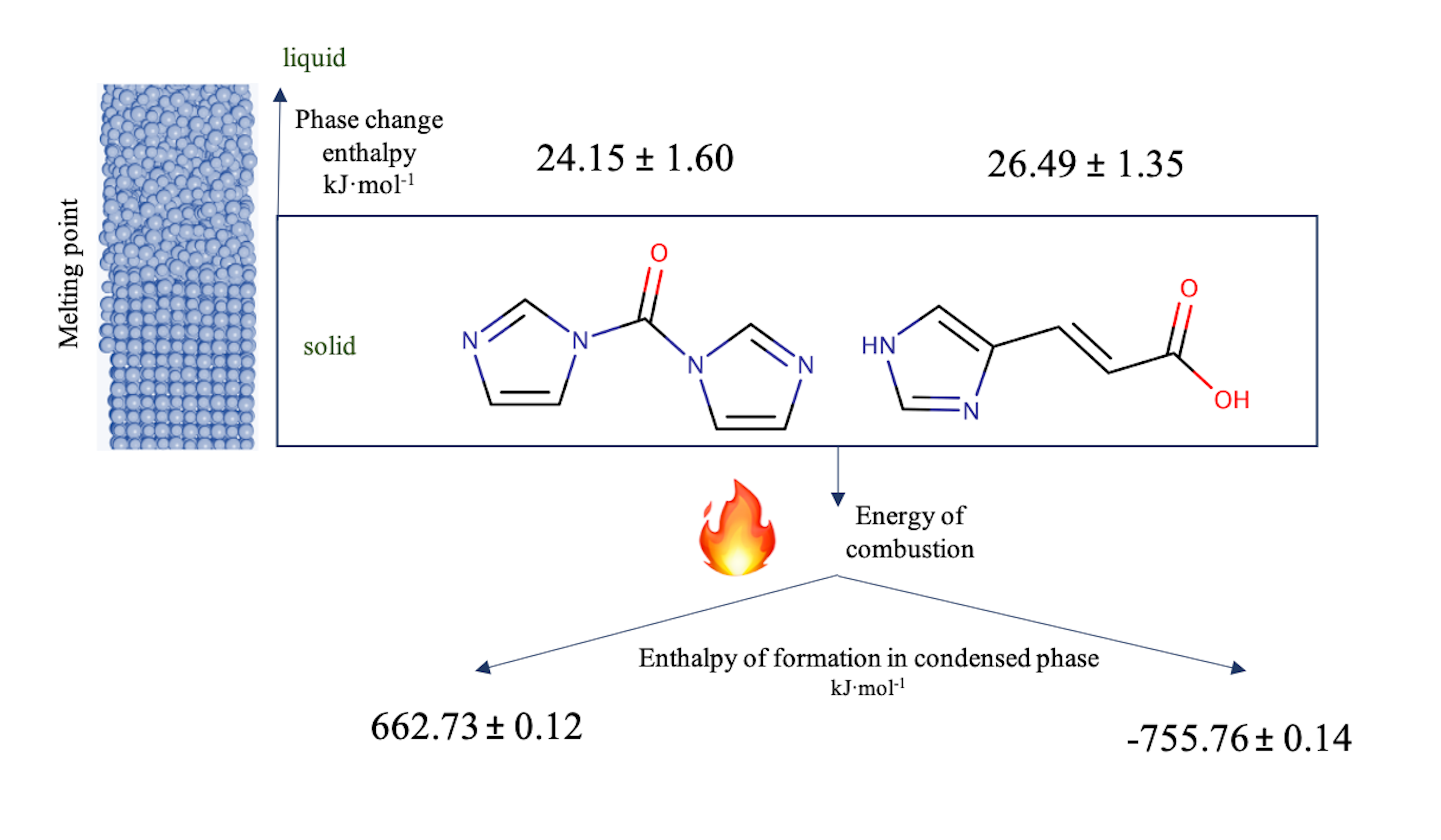
Abstract. In this work, thermodynamic properties of 1,1-carbonyldiimidazole (CDI) and 4-imidazole acrylic are reported. The melting temperature, the enthalpy of fusion and the heat capacity of the compounds were determined by differential scanning calorimetry. The standard molar energy of combustion of both compounds were determined by static-bomb combustion calorimetry and the standard molar enthalpy of formation in the crystalline phase, at T = 298.15 K, was derived and evaluated for the two imidazole derivatives studied. The energetic influence of the acrylic group on the imidazole ring in each of the properties obtained is analyzed and compared with the existing results in the literature.
Resumen. Se presentan las propiedades termodinámicas del 1,1-carbonildiimidazol (CDI) y el 4-imidazol acrílico. La temperatura de fusión, la entalpía de fusión y la capacidad calorífica de los compuestos se determinaron mediante calorimetría diferencial de barrido. La energía molar estándar de la combustión de ambos compuestos se determinó mediante calorimetría de combustión en bomba estática y la entalpía de formación en fase cristalina, a T= 298.15, fue derivada y evaluada para los dos compuestos derivados del imidazol. La influencia energética del grupo acrílico sobre el anillo de imidazol en cada una de las propiedades obtenidas se analiza y compara con los resultados existentes en la literatura.
Downloads
References
L. Wiseman, J. G. Lombardino, and E. H. Wiseman, “Preparation and Antiinflammatory Activity of Some Nonacidic Trisubstituted Imidazoles,” J. Med. Chem., vol. 17, no. 15, pp. 1182–1188, 1974. DOI: https://doi.org/10.1021/jm00257a011
R. J. Sundberg and R. B. Martin, “Interactions of Histidine and Other Imidazole Derivatives with Transition Metal Ions in Chemical and Biological Systems,” Chem. Rev., vol. 74, pp. 471–517, 1973. DOI: https://doi.org/10.1021/cr60290a003
K. Shalini, P. K. Sharma, and N. Kumar, “Imidazole and its biological activities : A review,” Chem. Sin., vol. 1, pp. 36–47, 2010.
A. S. Suvarna, “Imidazole and its derivatives and Importance in the Synthesis of Pharmaceuticals : A Review,” J. Chem. Sci., vol. 5, no. 10, pp. 67–72, 2015.
A. Verma, S. Joshi, and D. Singh, “Imidazole : Having Versatile Biological Activities,” J. Chem., vol. 2013, pp. 1–12, 2013. DOI: https://doi.org/10.1155/2013/329412
J. Fortún Abete, “No Title,” Medicine (Baltimore)., vol. 7, no. 91, pp. 133–140, 1998.
N. K. Gibbs and M. Norval, “Recent advances in urocanic acid photochemistry , photobiology and photoimmunology,” Photochem. Photobiol. Sci., vol. 7, pp. 655–667, 2008. DOI: https://doi.org/10.1039/b717398a
I. R. Scott, “Factors controlling the expressed activity of histidine ammonia-lyase in the epidermis and the resulting accumulation of urocanic acid,” Biochem. J., vol. 194, pp. 829–838, 1981. DOI: https://doi.org/10.1042/bj1940829
K. M. Hanson, B. Li, and J. D. Simon, “A Spectroscopic Study of the Epidermal Ultraviolet Chromophore trans -Urocanic Acid,” J. Am. Chem. Soc., vol. 119, pp. 2715–2721, 1997. DOI: https://doi.org/10.1021/ja963440s
T. Mohammad, H. Morrison, and H. Hogenesch, “Invited Review Urocanic Acid Photochemistry and Photobiology,” vol. 69, no. 2, 1999. DOI: https://doi.org/10.1562/0031-8655(1999)069<0115:UAPAP>2.3.CO;2
A. T. Using and A. L. Weber, “Aqueous synthesis of peptide thioesters from amino acids and a thiol using 1,1 -carbonyldiimidazole,” Orig Life Evol Biosph, vol. 35, pp. 421–422, 2005. DOI: https://doi.org/10.1007/s11084-005-4070-0
C. Plato, “Differential Scanning Calorimetry as a General Method for Determining Purity and Heat of Fusion of High- Purity Organic Chemicals,” Anal. Chem., vol. 44, no. 8, pp. 1531–1534, 1972. DOI: https://doi.org/10.1021/ac60316a049
J. Morales, G. Günther, and A. L. Zanocco, “Rapid and Simple HPLC Method for the Simultaneous Determination of Urocanic Acid Isomers in Human Skin,” Anal. Lett., vol. 46, no. October 2013, pp. 37–41, 2012. DOI: https://doi.org/10.1080/00032719.2012.706845
R. Sabbah, A. Xu-wu, J. S. Chickos, M. L. Planas Leitao, M. V. Roux, and L. A. Torres, “Reference materials for calorimetry and differential thermal analysis,” Thermochim. Acta, vol. 331, pp. 93–204, 1999. DOI: https://doi.org/10.1016/S0040-6031(99)00009-X
G. Höne, H. Hemminger, and H.-J. Flammersheim, Differential Scanning Calorimetry An Introduction for Practitioners, 1st ed. New York, 1996. DOI: https://doi.org/10.1007/978-3-662-03302-9_1
J. B. Campos, A. J. Martinez-Gómez, and E. Orozco-Guareno, “Design and building of an isoperibolic calorimeter: Measurements of enthalpy of formation for derivatives of glycidol,” Meas. Sci. Technol., vol. 30, no. 3, pp. 1–14, 2019. DOI: https://doi.org/10.1088/1361-6501/aafeb4
E. J. Prosen, “Combustion in a Bomb of Compounds Containing Carbon, Hydrogen, Oxygen, and Nitrogen,” in Experimental Thermochemistry Measurement of Heats of Reaction, 1st ed., New York: Interscience Publishers Inc., 1956, p. 141.
CODATA, “CODATA recommended key values for thermodynamics. Report of the CODATA Task Gropu on key values for thermodynamics.,” J. Chem. Thermodyn., vol. 10, pp. 903–906, 1978. DOI: https://doi.org/10.1016/0021-9614(78)90050-2
W. D. Good and N. K. Smith, “Enthalpies of combustion of toluene, benzene, cyclohexane, cyclohexene, methylcydopentane, 1-methylcyclopentene, and n-hexane,” J. Chem. Eng. Data, vol. 14, no. 1, pp. 102–106, 1969. DOI: https://doi.org/10.1021/je60040a036
J. Meija et al., “Atomic weights of the elements 2013 (IUPAC Technical Report),” Pure Appl. Chem., vol. 88, no. 3, pp. 265–291, 2016. DOI: https://doi.org/10.1515/pac-2015-0305
E. W. Washburn, “Standard States for bomb Calorimetry,” Bur. Stand. J. Res., vol. 10, no. 4, pp. 525–558, 1933. DOI: https://doi.org/10.6028/jres.010.037

Downloads
Additional Files
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









