Phycocyanin Thermo-photostability: an Accelerated Life-test Analysis
DOI:
https://doi.org/10.29356/jmcs.v64i3.1157Keywords:
Food coloring, microalgae, natural pigment, blue pigmentAbstract
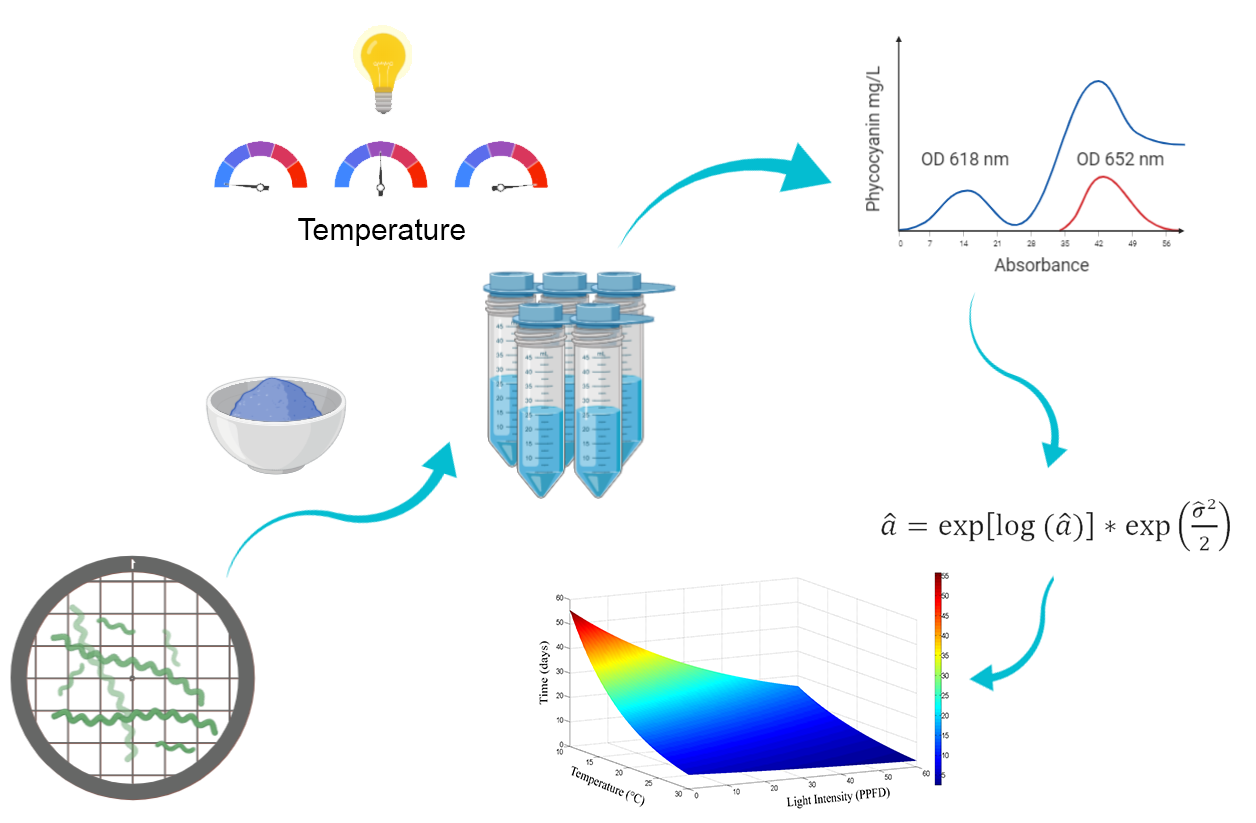
Abstract. Phycocyanin is a natural blue colorant with antioxidant activity which can be safely used in food, however its rapid degradation is still a concern for food manufacturing. Phycocyanin is easily degraded when exposed to mid-temperatures and/or light. Several studies have been stablished the degradation kinetics of aqueous solutions evaluating temperature or light as accelerating factors using a first order kinetic model and, both factors have been studied by separate or fixing one of them to evaluate the combined effect. The aim of this work was to develop an empirical model able to predict the effect of temperature and light combined in the degradation ratio of this pigment at selected storage conditions. We have tested five correlation models to fit temperature, light and time data to the degradation ratio of the phycocyanin; these were statistically tested to select the more appropriate. This is a novelty in the study of accelerated life-test analysis of phycocyanin, since most of the models are based on one accelerating variable at the time and the relationship between accelerating variables has not been explored before. We were able to develop a methodology to evaluate the effect of two accelerating life factors at once using CPC as model which is highly precise and easy to apply.
Resumen. La ficocianina es un pigmento natural color azul con actividad antioxidante que puede utilizarse de manera segura en alimentos, sin embargo, su rápida degradación sigue siendo un problema para su uso en alimentos. La ficocianina se degrada fácilmente cuando se expone a temperaturas medias o a la luz. Algunos estudios han establecido la cinética de degradación de las soluciones evaluando la temperatura o la luz como factores de aceleración usando modelos cinéticos de primer orden. Además, ambos factores han sido estudiados por separado o fijando uno de ellos para evaluar el efecto combinado. El objetivo de este trabajo fue desarrollar un modelo empírico capaz de predecir el efecto de la temperatura y la iluminación en forma combinada sobre la velocidad de degradación de la ficocianina a las condiciones de almacenamiento seleccionadas. Se probaron cinco modelos de correlación para ajustar los datos de temperatura, luz y tiempo a la velocidad de degradación de la ficocianina; dichos modelos fueron probados estadísticamente para determinar el más adecuado. Esta es una novedad en el estudio de los análisis de pruebas de vida acelerada de la ficocianina, dado que la mayoría de los modelos se basan en una sola variable acelerante a la vez y, no se han explorado las relaciones entre las variables de aceleración. Fuimos capaces de desarrollar una metodología altamente precisa y sencilla para evaluar el efecto de dos factores simultáneos de aceleración de la vida de la ficocianina C como modelo.
Downloads
References
(2) Antelo, F. S.; Costa, J. A. V.; Kalil, S. J. Thermal Degradation Kinetics of the Phycocyanin from Spirulina Platensis. Biochem. Eng. J. 2008, 41 (1), 43–47. https://doi.org/10.1016/j.bej.2008.03.012.
(3) González-Ramírez, E.; Andújar-Sánchez, M.; Ortiz-Salmerón, E.; Bacarizo, J.; Cuadri, C.; Mazzuca-Sobczuk, T.; Ibáñez, M. J.; Cámara-Artigas, A.; Martínez-Rodríguez, S. Thermal and PH Stability of the B-Phycoerythrin from the Red Algae Porphyridium Cruentum. Food Biophys. 2014, 9 (2), 184–192. https://doi.org/10.1007/s11483-014-9331-x.
(4) Kannaujiya, V. K.; Sinha, R. P. Thermokinetic Stability of Phycocyanin and Phycoerythrin in Food-Grade Preservatives. J. Appl. Phycol. 2016, 28 (2), 1063–1070. https://doi.org/10.1007/s10811-015-0638-x.
(5) Gouveia, L.; Batista, A. P.; Sousa, I.; Raymundo, A.; Bandarra, N. M. Microalgae in Novel Food Products. In Food Chemistry Research Developments; Papadopoulos, K. N., Ed.; Nova Publishers, 2008; pp 75–112.
(6) Priyadarshani, I.; Rath, B. Commercial and Industrial Applications of Micro Algae – A Review. J. Algal Biomass Util. 2012, 3 (4), 89–100. https://doi.org/10.1016/j.(73).
(7) Liang, Y.; Kaczmarek, M. B.; Kasprzak, A. K.; Tang, J.; Shah, M. M. R.; Jin, P.; Klepacz-Smółka, A.; Cheng, J. J.; Ledakowicz, S.; Daroch, M. Thermosynechococcaceae as a Source of Thermostable C-Phycocyanins: Properties and Molecular Insights. Algal Res. 2018, 35 (August), 223–235. https://doi.org/10.1016/j.algal.2018.08.037.
(8) Borowitzka, M. A. High-Value Products from Microalgae-Their Development and Commercialisation. J. Appl. Phycol. 2013, 25 (3), 743–756. https://doi.org/10.1007/s10811-013-9983-9.
(9) D’Alessandro, E. B.; Antoniosi Filho, N. R. Concepts and Studies on Lipid and Pigments of Microalgae: A Review. Renew. Sustain. Energy Rev. 2016, 58, 832–841. https://doi.org/10.1016/j.rser.2015.12.162.
(10) Sarada, R.; Pillai, M. G.; Ravishankar, G. . Phycocyanin from Spirulina Sp: Influence of Processing of Biomass on Phycocyanin Yield, Analysis of Efficacy of Extraction Methods and Stability Studies on Phycocyanin. Process Biochem. 1999, 34 (8), 795–801. https://doi.org/10.1016/S0032-9592(98)00153-8.
(11) Choi, W. Y.; Lee, H. Y. Kinetic Analysis of Stabilizing C-Phycocyanin in the Spirulina Platensis Extracts from Ultrasonic Process Associated with Effects of Light and Temperature. Appl. Sci. 2018, 8 (9). https://doi.org/10.3390/app8091662.
(12) Jespersen, L.; Strømdahl, L. D.; Olsen, K.; Skibsted, L. H. Heat and Light Stability of Three Natural Blue Colorants for Use in Confectionery and Beverages. Eur. Food Res. Technol. 2005, 220 (3–4), 261–266. https://doi.org/10.1007/s00217-004-1062-7.
(13) Wu, H. L.; Wang, G. H.; Xiang, W. Z.; Li, T.; He, H. Stability and Antioxidant Activity of Food-Grade Phycocyanin Isolated from Spirulina Platensis. Int. J. Food Prop. 2016, 19 (10), 2349–2362. https://doi.org/10.1080/10942912.2015.1038564.
(14) Colla, L. M.; Bertol, C. D.; Ferreira, D. J.; Bavaresco, J.; Costa, J. A. V.; Bertolin, T. E. Thermal and Photo-Stability of the Antioxidant Potential of Spirulina Platensis Powder. Brazilian J. Biol. 2017, 77 (2), 332–339. https://doi.org/10.1590/1519-6984.14315.
(15) Escobar, L. A.; Meeker, W. Q. A Review of Accelerated Test Models. Stat. Sci. 2006, 21 (4), 552–577. https://doi.org/10.1214/088342306000000321.
(16) Liu, Y.; Feng, Y.; Lun, J. Aqueous Two-Phase Countercurrent Distribution for the Separation of c-Phycocyanin and Allophycocyanin from Spirulina Platensis. Food Bioprod. Process. 2012, 90 (2), 111–117. https://doi.org/10.1016/j.fbp.2011.08.002.
(17) Pan-utai, W.; Kahapana, W.; Iamtham, S. Extraction of C-Phycocyanin from Arthrospira (Spirulina) and Its Thermal Stability with Citric Acid. J. Appl. Phycol. 2018, 30 (1), 231–242. https://doi.org/10.1007/s10811-017-1155-x.
(18) Montgomery, D.; Peck, E.; Vining, G. Introduction to Linear Regression Analysis, 5th ed.; Wiley: Hoboken, NJ, USA, 2012.
(19) Newman, M. C. Regression Analysis of Log-Transformed Data: Statistical Bias and Its Correction. Environ. Toxicol. Chem. 1993, 12 (6), 1129–1133. https://doi.org/10.1002/etc.5620120618.
(20) Braga, A. R. C.; Figueira, F. da S.; Silveira, J. T. da; Morais, M. G. de; Costa, J. A. V.; Kalil, S. J. Improvement of Thermal Stability of C-Phycocyanin by Nanofiber and Preservative Agents. J. Food Process. Preserv. 2016, 40 (6), 1264–1269. https://doi.org/10.1111/jfpp.12711.
(21) Chentir, I.; Hamdi, M.; Li, S.; Doumandji, A.; Markou, G.; Nasri, M. Stability, Bio-Functionality and Bio-Activity of Crude Phycocyanin from a Two-Phase Cultured Saharian Arthrospira Sp. Strain. Algal Res. 2018, 35 (May), 395–406. https://doi.org/10.1016/j.algal.2018.09.013.
(22) Sili, C.; Torzillo, G.; Vonshak, A. Arthrospira (Spirulina). In Ecology of Cyanobacteria II; Whitton, B. A., Ed.; Springer Netherlands: Dordrecht, 2012; pp 677–705. https://doi.org/10.1007/978-94-007-3855-3_25.

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









