Anti-inflammatory Activity of Piperlotines
DOI:
https://doi.org/10.29356/jmcs.v64i3.1152Keywords:
Mechanochemistry, piper amides, inflammation, tuberculosisAbstract
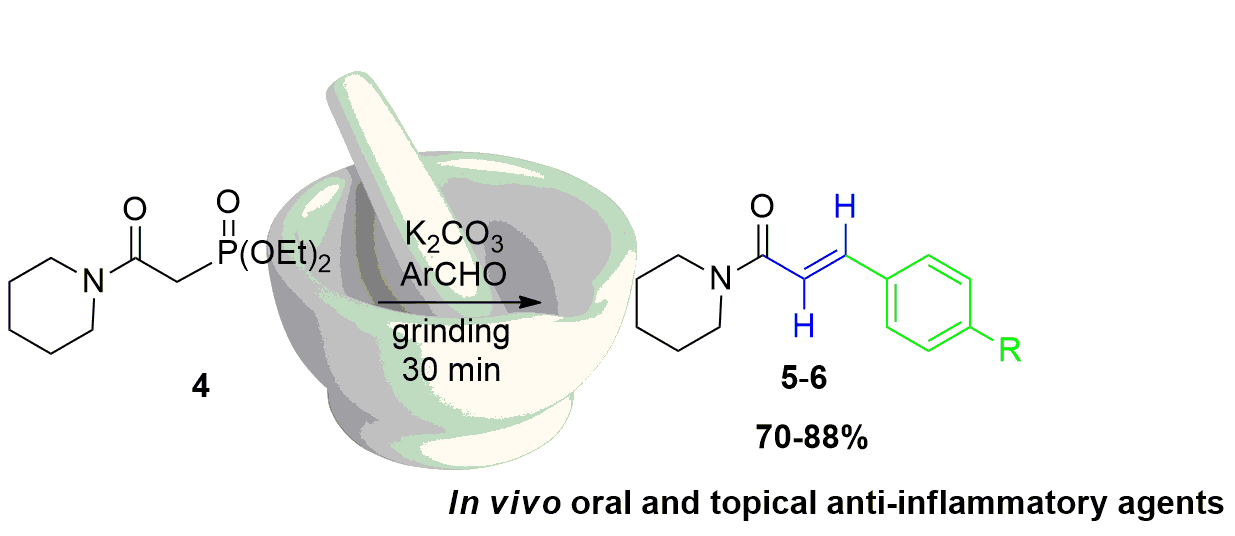
Abstract. In the present study we evaluated six α,β-unsaturated amides named piperlotines (for being isolated originally from Piper species) as new in vivo anti-inflammatory agents. In addition, we report the mechanosynthesis of two of them by mechanochemical activation of a Horner-Wadsworth-Emmons reaction. The reaction of β-amidophosphonate 4, an aromatic aldehyde and K2CO3 under grinding in a mortar and pestle afforded piperlotines 5-6 in good yields (70-88%) in short reaction times, obtaining only (E)-diastereomer. Piperlotines previously prepared were tested as anti-inflammatory and antibacterial agents. In this respect, derivatives 2 and 6 exhibited excellent in vivo anti-inflammatory activity on mice, especially trough topical administration (TPA acute inflammation model). Furthermore, piperlotine A, and compounds 2 and 6 had slight antimycobacterial activity against Mycobacterium tuberculosis (MIC = 50 µg/mL). In conclusion, the solvent-free mechanosynthesis of piperlotines produced valuable compounds that could serve as templates for further investigation in the search of better drug-like compounds for the treatment of inflammatory diseases.
Resumen. En la presente investigación se evaluó la actividad anti-inflamatoria in vivo de seis amidas α,β-insaturadas, identificadas en la literatura como piperlotinas debido a su inicial aislamiento a partir de especies vegetales del género Piper (como las pimientas). La reacción del β-amidofosfonato 4 con un aldehído aromático y K2CO3 en molienda con mortero y pistilo produjo las piperlotinas 5-6 con buenos rendimientos (70-80%) en tiempos cortos de reacción, obteniendo solamente el diastereoisómero (E). Las piperlotinas preparadas se evaluaron como agentes anti-inflamatorios y antibacterianos, observando excelente actividad anti-inflamatoria para los derivados 2 y 6, especialmente cuando se probaron mediante administración tópica (modelo de inflamación aguda por TPA). Además, la piperlotina A y los derivados 2 y 6 mostraron actividad antibacteriana contra Mycobacterium tuberculosis (MIC = 50 µg/mL). En conclusión, la síntesis de piperlotinas a través de molienda en condiciones libres de disolvente generó importantes productos que pueden ser utilizados como punto de partida para generar nuevos compuestos bioactivos para el tratamiento de padecimientos relacionados con la inflamación.
Downloads
References
Gómez?Calvario, V.; Rios, M.Y. Magn. Reson. Chem. 2019, 57, 1–77 DOI: https://doi.org/10.1002/mrc.4857
Li C-Y.; Tsai, W-J.; Damu, A. G.; Lee, E-J.; Wu, T-S.; Dung, N. X.; Thang, T. D.; Thanh, L. J. Agric. Food Chem. 2007, 55, 9436–9442 DOI: https://doi.org/10.1021/jf071963l
Hou, T.; Liao, N.; Xu, X.; Li, Y. J. Mol. Mod. 2000, 6, 438–445 DOI: ://doi.org/10.1007/s0089400060438 DOI: https://doi.org/10.1007/s0089400060438
Hou, T. J.; Wang, J. M.; Liao, N.; Xu, X. J. J. Chem. Inf. Comput. Sci. 1999, 39, 775–781 DOI: https://doi.org/10.1021/ci990010n
Alinaghi, F.; Calov, M.; Kristensen, L. E.; Gladman, D. D.; Coates, L. C.; Jullien, D.; Gottlieb, A. B.; Gisondi, P.; Wu, J. J.; Thyssen, J. P.; Egeberg, A. J. Am. Acad. Dermatol. 2019, 80, 251–265 DOI: https://doi.org/10.1016/j.jaad.2018.06.027
Barbour, K. E.; Helmick, C. G.; Boring, M.; Brady, T. J. MMWR Morb. Mortal Wkly. Rep. 2017, 66, 246–253 DOI: http://dx.doi.org/10.15585/mmwr.mm6609e1 DOI: https://doi.org/10.15585/mmwr.mm6609e1
The Global Asthma Report 2018. Auckland, New Zealand: Global Asthma Network, 2018.
World Health Organization (2019) Global tuberculosis report 2019. Geneva: 2019. Licence: CC BY-NC-SA 3.0 IGO.
Kakwani, M. D.; Suryavanshi, P.; Ray, M.; Rajan, M.; Majee, S.; Samad, A.; Devarajan, P.; Degani, M. S., Bioorganic & medicinal chemistry letters 2011, 21, 1997–1999 DOI: 10.1016/j.bmcl.2011.08.076 DOI: https://doi.org/10.1016/j.bmcl.2011.02.022
Wu, Z.-R.; Zhi, D.-J.; Zheng, L.-F.; Li, J.-Y.; Li, Y.; Xie, Q.-J.; Feng, N.; Bao, Y.-F.; Gao, Q.-Y.; Song, Y., Medicinal Chemistry Research 2015, 24, 161–170 DOI: 10.1007/s00044-014-1112-z DOI: https://doi.org/10.1007/s00044-014-1112-z
Rastogi, N.; Goh, K. S.; Horgen, L.; Barrow, W. W., FEMS Immunology & Medical Microbiology 1998, 21, 149–157. DOI: 10.1111/j.1574-695X.1998.tb01161.x DOI: https://doi.org/10.1111/j.1574-695X.1998.tb01161.x
Carvalho, S. A.; da Silva, E. F.; de Souza, M. V.; Lourenço, M. C.; Vicente, F. R., Bioorganic & medicinal chemistry letters 2008, 18, 538-541 DOI: 10.1016/j.bmcl.2007.11.091 DOI: https://doi.org/10.1016/j.bmcl.2007.11.091
Driller, K. M.; Prateeptongkum, S.; Jackstell, R.; Beller, M. Angew. Chem. Int. Ed. Engl. 2011, 50, 537–541 DOI: 10.1002/anie.201005823 DOI: https://doi.org/10.1002/anie.201005823
Gowri, P. M.; Radhakrishnan, S. V. S.; Rao, V. R. S.; Madnu, A.; Hymavathi, A.; Rao, J. M. Curr. Sci. 2015, 109, 1775–1777
Tavares-da-Silva, E. J.; Varela, C. L.; Pires, A. S.; Encarnação, J. C.; Abrantes, A. M.; Botelho, M. F.; Carvalho, R. A.; Proença, C.; Freitas, M.; Fernandes, E.; Roleira, F. M. F. Bioorg. Med. Chem. 2016, 24, 3556–3564 DOI: 10.1016/j.bmc.2016.05.065 DOI: https://doi.org/10.1016/j.bmc.2016.05.065
da Silva-Carrara, V.; Ferreira-Cunha-Júnior, E.; Torres-Santos, E. C.; Gonçalves-Correa, A.; Monteiro, J. L.; Galhardo-Demarchi, I.; Campana-Lonardoni, M. V.; Garcia-Cortez, D. A. Braz. J. Pharmacog. 2013, 23, 447–454. DOI: 10.1590/S0102-695X2013005000022 DOI: https://doi.org/10.1590/S0102-695X2013005000022
Guan, L.-P.; Wei, C.-X.; Deng, X.-Q.; Sui, X.; Piao, H.-R.; Quan, Z.-S. Eur. J. Med. Chem. 2009, 44, 3654–3657 DOI: 10.1016/j.ejmech.2009.02.015 DOI: https://doi.org/10.1016/j.ejmech.2009.02.015
Kim, S.; Lim, C.; Lee, S.; Lee, S.; Cho, H.; Lee, J.-Y.; Shim, D.-S.; Park, H. D.; Kim, S. ACS Comb. Sci. 2013, 15, 208–215 DOI: 10.1021/co400003d DOI: https://doi.org/10.1021/co400003d
Metro, T. X.; Bonnamour, J.; Reidon, T.; Sarpoulet, J.; Martinez, J.; Lamaty, F., Chemical communications 2012, 48, 11781-3 DOI: 10.1039/c2cc36352f DOI: https://doi.org/10.1039/c2cc36352f
Tan, D.; Strukil, V.; Mottillo, C.; Friscic, T., Chemical communications 2014, 50, 5248-50 DOI: https://doi.org/10.1039/c3cc47905f
Ramírez-Marroquín, O. A.; Manzano-Pérez, F.; López-Torres, A.; Hernández-López, A.; Cortés-Pacheco, A.; Reyes-González, M. A. Synth. Commun. 2019, 49, 244–255 DOI: 10.1080/00397911.2018.1550204 DOI: https://doi.org/10.1080/00397911.2018.1550204
Leung, P. S-W.; Teng, Y.; Toy, P. H. Org. Lett. 2010, 12, 4996–4999 DOI: 10.1021/ol1021614 DOI: https://doi.org/10.1021/ol1021614
Nguta, J. M.; Appiah-Opong, R.; Nyarko, A. K.; Yeboah-Manu, D.; Addo, P. G. A.; Otchere, I.; Kissi-Twum, A. J. Ethnopharmacol. 2016, 182, 10–15 DOI: 10.1016/j.jep.2016.02.010. DOI: https://doi.org/10.1016/j.jep.2016.02.010
Dzul-Beh, A. J.; García-Sosa, K.; Uc-Cachón, A. H.; Bórquez, J.; Loyola, L. A.; Barrios-García, H. B.; Peña-Rodríguez, L. M.; Molina-Salinas, G. M. Braz. J. Pharmacog. 2019, 29, 798–800 DOI: 10.1016/j.bjp.2019.06.001 DOI: https://doi.org/10.1016/j.bjp.2019.06.001
Do?an, H.; Do?an, S. D.; Gündüz, M. G.; Krishna, V. S.; Lherbet, C.; Sriram, D.; Sahin, O.; Saripinar, E. Eur. J. Med. Chem. 2020, 188, 112035 DOI: 10.1016/j.ejmech.2020.112035 DOI: https://doi.org/10.1016/j.ejmech.2020.112035
Pérez-González, M. Z.; Gutiérrez-Rebolledo, G. A.; Yépez-Mulia, L.; Rojas-Tomé I. S.; Luna-Herrera, J.; Jiménez-Arellanes, M. A. Biomed. Pharmacother. 2017, 89, 89–97 DOI: 10.1016/j.biopha.2017.02.021 DOI: https://doi.org/10.1016/j.biopha.2017.02.021
Solanki, H. K.; Shah, D. A.; Maheriya, P. M.; Patel, C. A. Int. J. Biol. Macromol. 2015, 72, 1277–1282 DOI: 10.1016/j.ijbiomac.2014.09.059 DOI: https://doi.org/10.1016/j.ijbiomac.2014.09.059
Jisha, N.; Vysakh, A.; Vijeesh, V.; Latha, M. S. Pathophysiology 2019, 26, 323–330 DOI: 10.1016/j.pathophys.2019.08.002 DOI: https://doi.org/10.1016/j.pathophys.2019.08.002
Xu, X-T.; Mou, X-Q.; Xi, Q-M.; Liu, W-T.; Liu, W-F.; Sheng, Z-J.; Zheng, X.; Zhang, K.; Du, Z-Y.; Zhao, S-Q.; Wang, S-H. Bioorg. Med. Chem. Lett. 2016, 26, 5334–5339 DOI: 10.1016/j.bmcl.2016.09.034
Xu, X-T.; Mou, X-Q.; Xi, Q-M.; Liu, W-T.; Liu, W-F.; Sheng, Z-J.; Zheng, X.; Zhang, K.; Du, Z-Y.; Zhao, S-Q.; Wang, S-H. Bioorg. Med. Chem. Lett. 2016, 26, 5334–5339 DOI: 10.1016/j.bmcl.2016.09.034 DOI: https://doi.org/10.1016/j.bmcl.2016.09.034
Figueroa-Suárez, M. Z.; González-Christen, J.; Cardoso-Taketa, A. T.; Gutiérrez-Villafuerte, M. C.; Rodríguez-López, V. J. Ethnopharmacol. 2019, 238, 111786 DOI: 10.1016/j.jep.2019.03.013 DOI: https://doi.org/10.1016/j.jep.2019.03.013
Madrigal, D. A.; Escalante, C. H.; Gutiérrez-Rebolledo, G. A.; Cristobal-Luna, J. M.; Gómez-García, O.; Hernández-Benitez, R. I.; Esquivel-Campos, A. L.; Pérez-Gutiérrez, S.; Chamorro-Cevallos, G. A.; Delgado, F.; Tamariz, J. Bioorg. Med. Chem. 2019, 27, 115053 DOI: 10.1016/j.bmc.2019.115053 DOI: https://doi.org/10.1016/j.bmc.2019.115053
Druzhilovskiy, D.; Rudik, A.; Filimonov, D.; Lagunin, A.; Gloriozova, T.; Poroikov, V. Russ. Chem. Bull. 2016, 65, 384–393 DOI: 10.1007/s11172-016-1310-6 DOI: https://doi.org/10.1007/s11172-016-1310-6
Bang, J. S.; Oh, D. H.; Choi, H. M.; Sur, B-J.; Lim, S-J.; Kim, J. Y.; Yang, H-I.; Yoo, M. C.; Hahm, D-H.; Kim, K. S. Arthritis Res. Ther. 2009, 11:R49 DOI: 10.1186/ar2662 DOI: https://doi.org/10.1186/ar2662
Umar, S.; Sarwar, A. H. M. G.; Umar, K.; Ahmad, N.; Sajad, M.; Ahmad, S.; Katiyar, C. K.; Khan, H. A. Cell. Immunol. 2013, 284, 51–59. DOI: /10.1016/j.cellimm.2013.07.004 DOI: https://doi.org/10.1016/j.cellimm.2013.07.004
Lu, Y.; Liu, J.; Li, H.; Gu, L. Inflammation 2016, 39, 303–308. DOI: g/10.1007/s10753-015-0250-x DOI: https://doi.org/10.1007/s10753-015-0250-x
Dolfi, F.; Pilgrim, W. R. Use of idrocilamide for the preparation of a pharmaceutical composition of rosacea. PCT WO2005/060950A1, July 7, 2005.
Darakhshan, S.; Pour, A. B. Pharmacol. Res. 2015, 91, 15–28 DOI: 10.1016/j.phrs.2014.10.009 DOI: https://doi.org/10.1016/j.phrs.2014.10.009
Zhou, P.; Xiang, L.; Zhao, D.; Ren, J.; Qiu, Y.; Li, Y. Med. Chem. Commun. 2019, 10, 252–262 DOI: 10.1039/c8md00432c DOI: https://doi.org/10.1039/C8MD00432C
Nissinen, L.; Kähäri, V-M. Biochim. Biophys. Acta 2014, 1840, 2571–2580 DOI: 10.1016/j.bbagen.2014.03.007 DOI: https://doi.org/10.1016/j.bbagen.2014.03.007
Chang, Y-H.; Lin, I-L.; Tsai, G. J.; Yang, S-C.; Yang, T-P.; Ho, K-T.; Tsu, T-C.; Shiau, M-Y. Clin. Biochem. 2008, 41:955–959 DOI: 10.1016/j.clinbiochem.2008.04.012 DOI: https://doi.org/10.1016/j.clinbiochem.2008.04.012
Li, N.; Qiao, Y.; Xue, L.; Xu, S.; Zhang, N. Int. J. Pharm. 2019, 569, 118625 DOI: 10.1016/j.ijpharm.2019.118625 DOI: https://doi.org/10.1016/j.ijpharm.2019.118625
Wajant, H.; Pfizenmaier, K.; Scheurich, P. Cell Death Differ. 2003, 10, 45–65 DOI: 10.1038/sj.cdd.4401189 DOI: https://doi.org/10.1038/sj.cdd.4401189
Lipinski, C. A.; Lombardo, F.; Dominy, B. W.; Feeney, P. J. Adv. Drug Deliv. Rev. 1997, 23, 3–25 DOI: 10.1016/s0169-409x(96)00423-1 DOI: https://doi.org/10.1016/S0169-409X(96)00423-1
Shah, P.; Westwell, A. D. J. Enzyme Inhib. Med. Chem. 2007, 22, 527–540. DOI: 10.1080/14756360701425014 DOI: https://doi.org/10.1080/14756360701425014
Jeffries, B.; Wang, Z.; Graton, J.; Holland, S. D.; Brind, T.; Greenwood, R. D. R.; Le Questel, J-Y.; Scott, J. S.; Chiarparin, E.; Linclau, B. J. Med. Chem. 2018, 61, 10602–10618 DOI: 10.1021/acs.jmedchem.8b01222 DOI: https://doi.org/10.1021/acs.jmedchem.8b01222
Ramírez-Marroquín, O. A.; Jiménez-Arellanes, M. A.; Cortés-Pacheco, A.; Zambrano-Vásquez, O. R.; López-Torres, A. Monatsh. Chem. 2019, 150, 267–274 DOI: 10.1007/s00706-018-2328-2 DOI: https://doi.org/10.1007/s00706-018-2328-2
https://www.molinspiration.com/cgi-bin/properties, accessed in January 2020.
Reddy, V. M.; Nadadhur, G.; Daneluzzi, D.; Dimova, V.; Gangadharam, P. R. Antimicrob. Agents Chemother. 1995, 39, 2320–2324 DOI: 10.1128/AAC.39.10.2320 DOI: https://doi.org/10.1128/AAC.39.10.2320
Philipova, I.; Valcheva, V.; Mihaylova, R.; Mateeva, M.; Doytchinova, I.; Stavrakov, G. Chem. Biol. Drug Des. 2018, 91, 763–768. DOI: 10.1111/cbdd.13140 DOI: https://doi.org/10.1111/cbdd.13140
Tuntiwachwuttikul, P.; Phansa, P.; Pootaeng-on, Y.; Taylor, W. C. Chem. Pharm. Bull. 2006, 54, 149–151 DOI: 10.1248/cpb.54.149 DOI: https://doi.org/10.1248/cpb.54.149
Sharma, S.; Kumar, M.; Sharma, S.; Nargotra, A.; Koul, S.; Khan, I. A. J. Antimicrob. Chemother. 2010, 65, 1694–1701. DOI: 10.1093/jac/dkq186 DOI: https://doi.org/10.1093/jac/dkq186
Sharma, S.; Kalia, N. P.; Suden, P.; Chauhan, P. S.; Kumar, M.; Ram, A. B.; Khajuria, A.; Bani, S.; Khan, I. A. Tuberculosis 2014, 94, 389–396. DOI: 10.1016/j.tube.2014.04.007 DOI: https://doi.org/10.1016/j.tube.2014.04.007

Downloads
Additional Files
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









