Electrochemical Degradation of Metoprolol Using Graphite-PVC Composite as Anode: Elucidation and Characterization of New by-products Using LC-TOF/MS
DOI:
https://doi.org/10.29356/jmcs.v64i3.1139Keywords:
Metoprolol, electrochemical degradation, by-products, solid phase extraction, LC-TOF/MSAbstract
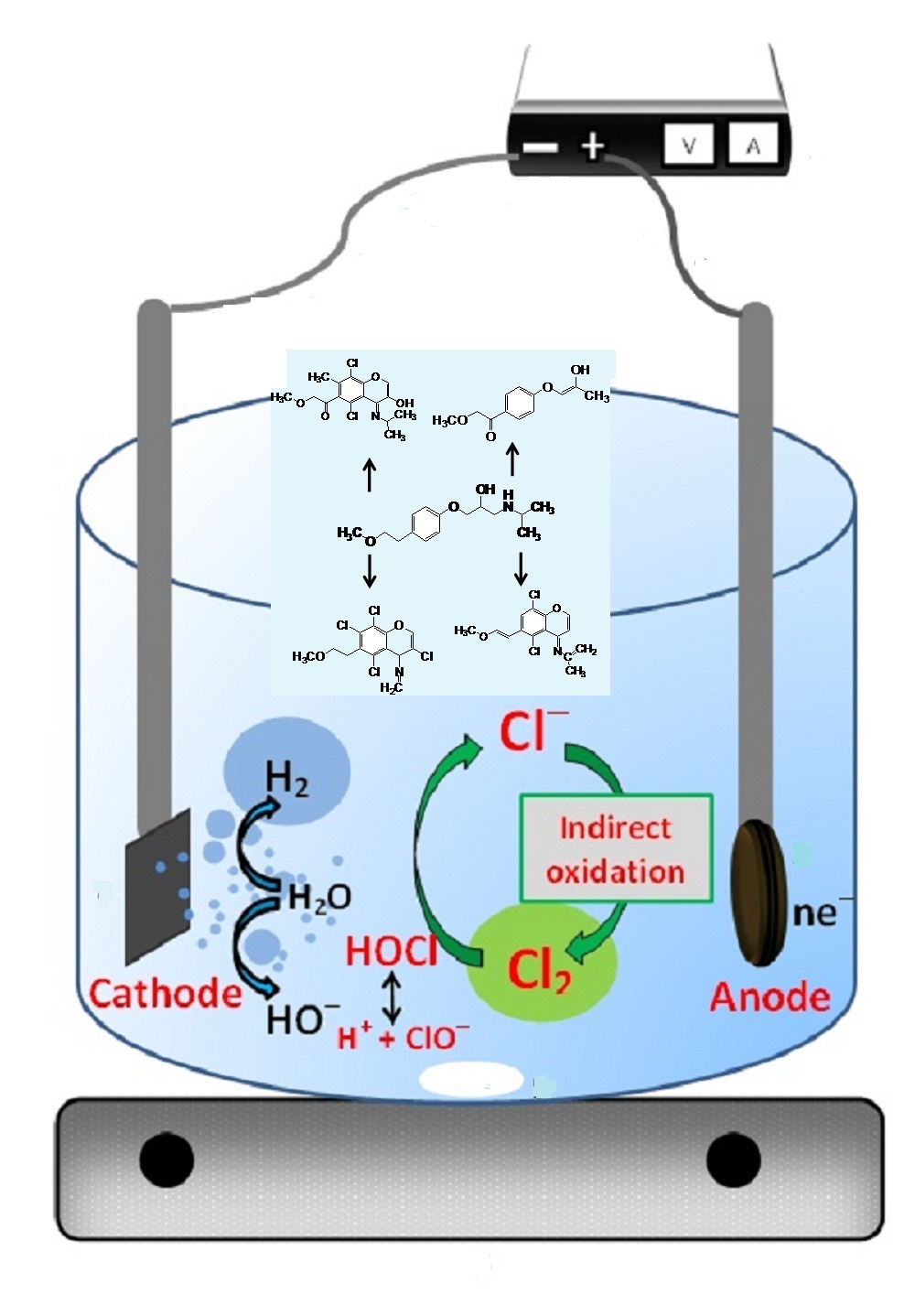
Abstract. Metoprolol (MTP) is one of pharmaceuticals used for treatment of heart failure and hypertension. It was frequently detected in wastewater samples either influent or effluent. The application of graphite-PVC composite as anode was investigated for the degradation of metoprolol in the presence of strong electrolyte such as sodium chloride (NaCl). The degradation rate was strongly influenced by initial concentrations of metoprolol, NaCl concentration and applied voltage. An initial concentration of 2 mg/L was eliminated more than 95% after 30 min under optimum conditions; 5000 mg/L NaCl and 5 V. The consumption energy of the electrochemical reaction was 0.665 Wh/mg for metoprolol after 30 min. The kinetic rate constant of metoprolol could be ranged between 0.0016 and 0.0801 min-1. The electrochemical degradation efficiency of metoprolol and its by-products has been achieved. The degradation of metoprolol produced four transformated products as investigated and elucidated using liquid chromatography-time of flight/mass spectrometry. The proposed degradation pathway of metoprolol was schemed on the base of the identified intermediates.
Resumen. El metoprolol (MTP) es uno de los fármacos utilizados para el tratamiento de la insuficiencia cardíaca y la hipertensión. Se detecta frecuentemente en muestras de aguas residuales, ya sea de afluentes o efluentes. Se investigó la aplicación del compuesto de grafito-PVC como ánodo para la degradación del metoprolol en presencia de un electrolito fuerte como el cloruro de sodio (NaCl). La velocidad de degradación depende de las concentraciones iniciales de metoprolol, la concentración de NaCl y el voltaje aplicado. Una concentración inicial de 2 mg/L de MTP fue eliminada con más del 95% después de 30 minutos en condiciones óptimas; 5000 mg/L de NaCl y 5 V. La energía de consumo de la reacción electroquímica fue de 0,665 Wh/mg para el metoprolol después de 30 min. La constante cinética de degradación metoprolol oscila entre 0.0016 y 0.0801 min-1. Se logró la eficiente degradación electroquímica del metoprolol y sus subproductos, ya que se detectaron cuatro subproductos electrogenerados según los resultados de cromatografía líquida - tiempo de vuelo/espectrometría de masas. La vía de degradación propuesta del metoprolol se esquematizó sobre la base de los productos intermedios identificados.
Downloads
References
Al-Odaini, N. A.; Zakaria, M. P.; Yaziz, M. I.; Surif, S. J. Chromatogr. A 2010,1217, 6791-6806. https://doi.org/10.1016/j.chroma.2010.08.033
Al-Qaim, F. F.; Abdullah, M. P.; Othman, M. R.; Latip, J.; Zakaria, Z. J. Chromatogr. A 2014, 1345, 139-153. https://doi.org/10.1016/j.chroma.2014.04.025
Al-Qaim, F. F.; Mussa, Z. H.; Yuzir, A. Anal. Bioanal. Chem. 2018, 410, 4829-4846. DOIhttps://doi.org/10.1007/s00216-018-1120-9
Kotowska, U.; Kapelewska, J.; Sturgulewska, J. Environ. Sci. Poll. Res. 2018, 21, 660-673. DOIhttps://doi.org/10.1007/s11356-013-1904-6
Valcárcel, Y.; Alonso, S. G.; Rodríguez-Gil, J. L.; Maroto, R. R.; Gil, A.; Catalá, M. Chemosphere 2011 82, 1062-1071. https://doi.org/10.1016/j.chemosphere.2010.10.041
You, L.; Nguyen, V. T.; Pal, A.; Chen, H.; He, Y.; Reinhard, M.; Gin, K. Y. H. Sci. Total Environ. 2015, 536, 955-963. https://doi.org/10.1016/j.scitotenv.2015.06.041
Anumol, T.; Clarke, B. O.; Merel, S.; Snyder, S. A. J. American Water Works Association, 2015, 107, E474-E485.https://doi.org/10.5942/jawwa.2015.107.0129
Feng, L.; Van Hullebusch, E. D.; Rodrigo, M. A.; Esposito, G.; Oturan, M. A. A review. Chem. Eng. J.2013, 228, 944-964. https://doi.org/10.1016/j.cej.2013.05.061
Nikolaou, A.; Meric, S.; Fatta, D. Anal. Bioanal. Chem. 2007, 387, 1225-1234. DOIhttps://doi.org/10.1007/s00216-006-1035-8
Zhang, Y.; Geißen, S. U.; Gal, C. Chemosphere 2008, 73, 1151-1161. https://doi.org/10.1016/j.chemosphere.2008.07.086
Al-Qaim, F. F.; Abdullah, P.; Othman, M. R.; Latip, J.; Afiq, W. M. Trop. J. Pharm. Res. 2013, 12, 609-616. http://dx.doi.org/10.4314/tjpr.v12i4.25 DOI: https://doi.org/10.4314/tjpr.v12i4.25
Drugbank. https://www.drugbank.ca/drugs/DB00264. Accessed 09 January 2020.
Holkar, C. R.; Jadhav, A. J.; Pinjari, D. V.; Mahamuni, N. M.; Pandit, A. B. J. Environ. Manag. 2016, 182, 351-366. https://doi.org/10.1016/j.jenvman.2016.07.090
Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Bio. Technol.2001, 77, 247-255. https://doi.org/10.1016/S0960-8524(00)00080-8
Martínez-Huitle, C. A.; Brillas, E. Appl. Catal. B: Environ. 2009, 87, 105-145. https://doi.org/10.1016/j.apcatb.2008.09.017
Riera-Torres, M.; Gutiérrez, M. C. Chem. Eng. J. 2010, 156, 114-120. https://doi.org/10.1016/j.cej.2009.10.006
Orts, F.; Del Río, A. I.; Molina, J.; Bonastre, J.; Cases, F. J. Electroanal. Chem. 2018, 808, 387-394. https://doi.org/10.1016/j.jelechem.2017.06.051
Luo, J.; Wang, Y.; Cao, D.; Xiao, K.; Guo, T.; Zhao, X. Chem. Eng. J.2018, 343, 69-77. https://doi.org/10.1016/j.cej.2018.02.120
Mussa, Z. H.; Al-Qaim, F. F.; Othman, M. R.; Abdullah, M. P.; Latip, J.; Zakria, Z. J. Taiwan Inst. Chem. Eng. 2017, 72, 37-44. https://doi.org/10.1016/j.jtice.2016.12.031
Barrera-Díaz, C.; Cañizares, P.; Fernández, F. J.; Natividad, R.; Rodrigo, M. A. J. Mex. Chem. Soc. 2014, 58, 256-275. https://doi.org/10.29356/jmcs.v58i3.133
Liu, Y. J.; Hu, C. Y.; Lo, S. L. J. Hazard. Mater. 2019, 366, 592-605. https://doi.org/10.1016/j.jhazmat.2018.12.037
Peters, D. G.; McGuire, C. M.; Pasciak, E. M.; Peverly, A. A.; Strawsine, L. M.; Wagoner, E. R.; Tyler B. J. J. Mex. Chem. Soc. 2014, 58, 287-302. https://doi.org/10.29356/jmcs.v58i3.135
Moreira, F. C.; Boaventura, R. A.; Brillas, E.; Vilar, V. J. Appl. Catal. B-Environ.2017, 202, 217-261. https://doi.org/10.1016/j.apcatb.2016.08.037
Mussa, Z. H.; Othman, M. R.; Abdullah, M. P. J. Brazil. Chem. Soc. 2015, 26, 939-948. http://dx.doi.org/10.5935/0103-5053.20150055 DOI: https://doi.org/10.5935/0103-5053.20150055
de Vidales, M. J. M.; Sáez, C.; Pérez, J. F.; Cotillas, S.; Llanos, J.; Canizares, P.; Rodrigo, M. A. J. Appl. Electrochem. 2015, 45, 799-808. https://doi.org/10.1007/s10800-015-0825-0
Al-Qaim, F. F.; Mussa, Z. H.; Othman, M. R.; Abdullah, M. P. J. Hazard. Mater. 2015, 300, 387-397. https://doi.org/10.1016/j.jhazmat.2015.07.007
Dubbelman, A. C.; Cuyckens, F.; Dillen, L.; Gross, G.; Vreeken, R. J.; Hankemeier, T. Anal. Chim. Acta 2018, 1020, 62-75. https://doi.org/10.1016/j.aca.2018.02.055
Zhu, H.; Lin, H.; Tan, J.; Wang, C.; Wang, H.; Wu, F.; Liu, J. Molecules, 2019, 24, 33. https://doi.org/10.3390/molecules24010033
Mussa, Z. H.; Al-Qaim, F. F.; Othman, M. R.; Abdullah, M. P. J. Environ. Chem. Eng. 2016, 4, 3338-3347. https://doi.org/10.1016/j.jece.2016.07.006
Deborde, M.; Von Gunten, U. R. S. Water Res.2008, 42, 13-51. https://doi.org/10.1016/j.watres.2007.07.025
Soufan, M.; Deborde, M.; Legube, B. Water Res. 2012, 46, 3377-3386. https://doi.org/10.1016/j.watres.2012.03.056
32 Olvera-Vargas, H.; Cocerva, T.; Oturan, N.; Buisson, D.; Oturan, M. A. J. Hazard. Mater. 2016, 319, 13-23. https://doi.org/10.1016/j.jhazmat.2015.12.010
33.Sirés, I.; Oturan, N.; Oturan, M. A. Water Res., 2010, 44, 3109-3120. https://doi.org/10.1016/j.watres.2010.03.005

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









