Cordination Compound of Dimethyltin(IV) with N,N,N’N’-Tetraethylethylenediamine: Speciation and Theoretical approach
DOI:
https://doi.org/10.29356/jmcs.v64i2.1079Keywords:
Dimethyltin(IV), N,N,N’,N’-tetraethylethylenediamine, stability constants, DFT calculationAbstract
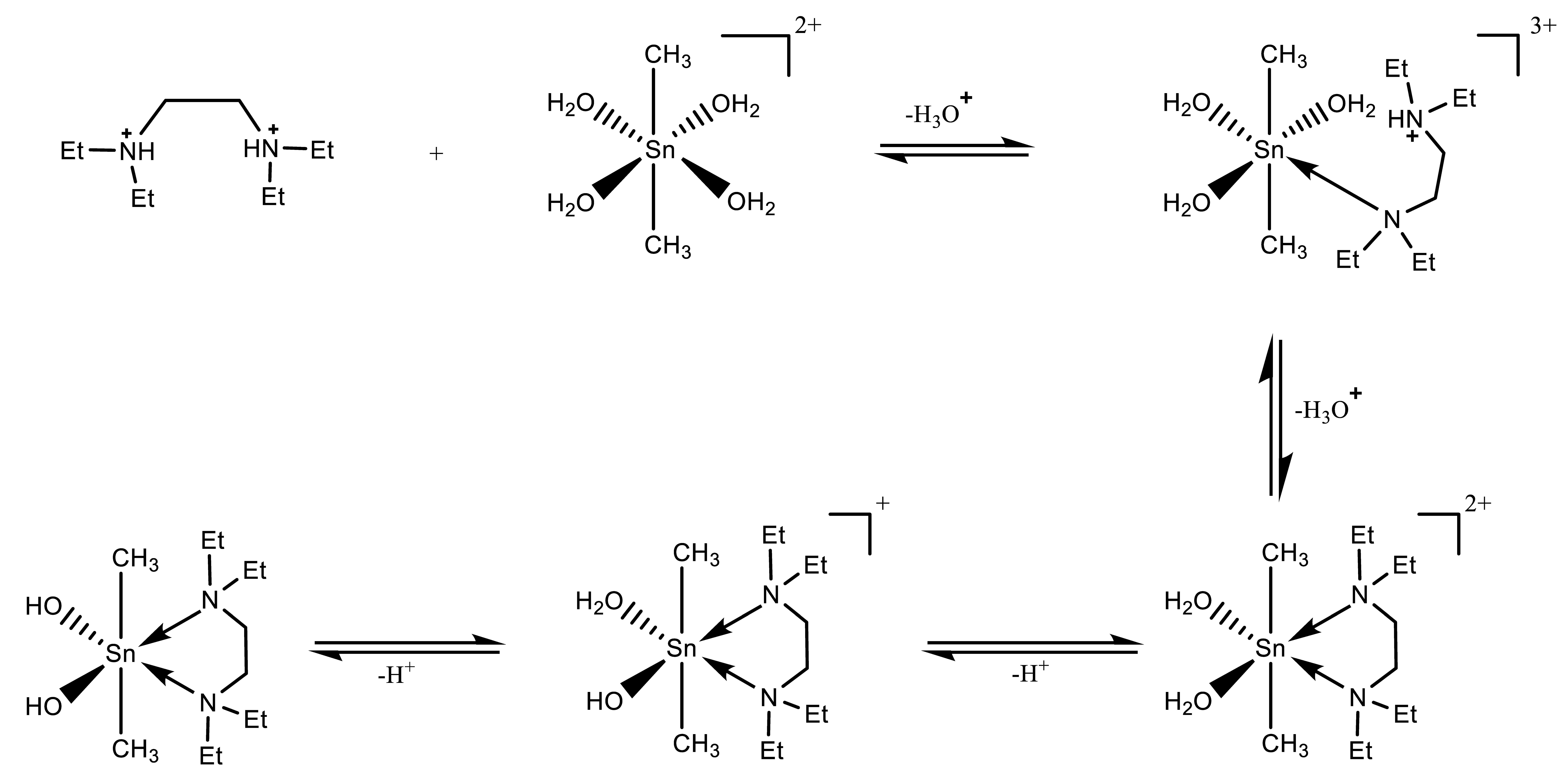
The formation equilibria of the dimethyltin(IV) complexes with of N,N,N’,N’-tetraethylethylenediamine (Et4en) in solution were investigated. The stoichiometry and stability constants of the complexes formed in solution phase were determined at different temperatures (15 oC – 35 oC) and in solutions of dioxane-water mixtures of different compositions (15% - 62.5%). The accepted model is composed of the 110, 111, 11-1 and 11-2 species. The thermodynamic parameters H and S associated with the protonation of N,N,N`,N`-tetraethylethylendiamine (Et4en) and its complex formation with the dimethyltin(IV) species were determined. The complex formation reaction is exothermic. The equilibrium constant for the displacement of N,N,N’,N’-tetraethylethylenediamine coordinated to dimethyltin(IV) by some selected DNA constituents was calculated. The Keq values clearly indicate the ability of DNA to displace the coordinated Et4en from its dimethyltin(IV) complex. The nucleotides IMP and GMP have the highest values. The DFT/B3LYP method was used for geometric optimization of the ligand and the complex using the Gaussian 09 program. Also the vibrational frequencies of the ligands and complexes were computed for the optimized geometries. The results shows that there is no imaginary frequencies as found in the calculated vibrational frequencies. The binding energies of the dimethyltin(IV) complexes were calculated. All calculated binding energy values are negative.
Downloads
References
Gasser, G.; Ott, I.; Metzler-Nolte, N. J. Med. Chem. 2011, 54, 3-25 DOI: https://doi.org/10.1021/jm100020w
Gianferrara, T.; Bratsos, I.; Alessio, E. Dalton Trans. 2009, 7588-7598 DOI: https://doi.org/10.1039/b905798f
Moody, L.; Holder, A. A. Annu. Rep. Prog. Chem., Sect. A 2009, 105, 505-524 DOI: https://doi.org/10.1039/b818288b
Hadjikakou, S.K.; Hadjiliadis, N. Coord. Chem. Rev. 2009, 253, 235-249 DOI: https://doi.org/10.1016/j.ccr.2007.12.026
Tabassum, S.; Pettinari, C. J. of Organomet. Chem. 2006, 691, 1761-1766 DOI: https://doi.org/10.1016/j.jorganchem.2005.12.033
Pellerito, C.; Nagy, L.; Pellerito, L.; Szorcsik, A. J. of Organomet. Chem. 2006, 691, 1733-174 DOI: https://doi.org/10.1016/j.jorganchem.2005.12.025
Gielen, M.; Biesemans, M.; Willem, R. Appl. Organometal. Chem. 2005; 19, 440-450 DOI: https://doi.org/10.1002/aoc.771
Pellerito, L.; Nagy, L. Coord. Chem. Rev.2002, 224, 111-150 DOI: https://doi.org/10.1016/S0010-8545(01)00399-X
Clarke, M. J.; Zhu, F.; Frasca, D. R. Chem. Rev. 1999, 99, 2511-2533 DOI: https://doi.org/10.1021/cr9804238
Gielen, M. Coord. Chem. Rev. 1996, 151, 41-51. DOI: https://doi.org/10.1016/S0010-8545(96)90193-9
Sadler, P.J. Adv. Inorg. Chem. 1991, 36, 1-48 DOI: https://doi.org/10.1016/S0898-8838(08)60035-5
Guo, Z.; Sadler, P.J. Angew. Chem. Int. Ed. 1999, 38, 1512-1531 DOI: https://doi.org/10.1002/(SICI)1521-3773(19990601)38:11<1512::AID-ANIE1512>3.0.CO;2-Y
Tlahuext?Aca, A.; Höpfl, H.; Medrano?Valenzuela, F.; Guerrero?Alvarez, J.; Tlahuext, H.; Ochoa Lara, K.; Reyes-Márquez, V.; Tlahuextl, M. Z. Anorg. Allg. Chem. 2012, 638, 1–9
Kotsakis, A.; Hatzidaki, D.; Vamvakas, L.; Vardakis, N.; Kalykaki, A.; Bozionelou, V.; Androulakis, N.; Kalbakis K.; Saridaki, Z.; Georgoulias, V. Anticancer Res., 2010, 30, 4335-4342 DOI: https://doi.org/10.1002/zaac.201200099
Gielen, M.; Willem, R. Anticancer Research, 1992, 12, 269-271. DOI: https://doi.org/10.1016/0920-1211(92)90082-5
Crowe, A. J. Antitumour activity of tin compounds, in: Metal Compounds in Cancer Therapy, Springer, 1994, 147-179. DOI: https://doi.org/10.1007/978-94-011-1252-9_7
Crowe, A. J.; Smith, P. J.; Cardin, C. J.; Parge, H. E.; Smith, F. E. Cancer Lett. 1984, 24, 45-48 DOI: https://doi.org/10.1016/0304-3835(84)90078-8
Gielen, M.; Meriem, A.; Boualam, M.; Willem, R. In vivo (Athens, Greece) 1993, 7, 171-174.
Yahyi, A.; Et-Touhami, A.; Yahyaoui, R.; Touzani, R. Lett. Drug Des. Discovery 2010, 7, 534-540. DOI: https://doi.org/10.2174/157018010791526241
Muhammad, N.; Shah, A.; Shuja, S.; Ali, S.; Qureshi, R.; Meetsma, A.; Tahir, M.N. J. Organomet. Chem. 2009, 694, 3431-3437 DOI: https://doi.org/10.1016/j.jorganchem.2009.06.036
Mohamed, M. M. A.; Shehata, M. R.; Shoukry, M. M. J. Coord. Chem. 2001, 53, 125-142 DOI: https://doi.org/10.1080/00958970108022607
Al Alousi, A. S.; Shehata, M. R.; Shoukry, M. M.; Mohamed, N. M. Chem. Speciation Bioavailability 2009, 21, 1-6 DOI: https://doi.org/10.3184/095422909X416216
Al-Najjar, A.; Mohamed, M. M. A.; Shehata, M. R.; Shoukry, M. M. Ann. Chim. (Rome, Italy) 2006, 96, 97-107 DOI: https://doi.org/10.1002/adic.200690010
Al-Najjar, A.; Mohamed, M. M. A.; Shoukry, M. M. J. of Coord. Chem. 2006, 59, 193-206 DOI: http://dx.doi.org/10.1080/00958970500270786 DOI: https://doi.org/10.1080/00958970500270786
Mohamed, M. M. A.; Shoukry, M. M. Chem. Pharm. Bull. 2001, 49, 253-257 DOI: https://doi.org/10.1248/cpb.49.253
Shehata, M. R.; Mohamed, M. M. A.; Shoukry, M. M.; Hussein, M. A.; Hussein, F. M. J. Coord. Chem. 2015, 68, 1101-1114 DOI: http://dx.doi.org/10.1080/00958972.2015.1007962 DOI: https://doi.org/10.1080/00958972.2015.1007962
27.Bates, R. Theory and Practice Wley, New York, Sydney (1964).
28.Motekaitis, R. J.; Martell, A. E.; Nelson, D. A. Inorg. Chem. 1984, 23, 275-283 DOI: https://doi.org/10.1021/ic00171a005
Shoukry, M. M.; Shehata, M. R.; Hamza, M. S.; van Eldik, R. Dalton Trans. 2005, 3921-3926 DOI: http://dx.doi.org/10.1039/b510553f DOI: https://doi.org/10.1039/b510553f
Gans, P.; Sabatini, A.; Vacca, A. Inorg. Chim. Acta 1976, 18, 237-239 DOI: https://doi.org/10.1016/S0020-1693(00)95610-X
L. Pettit, Personal communication, 1993 .
Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, G. Z. J.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A.; Peralta, Jr. J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R., Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Prich, S. D.; Daniels, A. D.; Farkas, O.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Wallingford CT, Gaussian Inc. 2009.
Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785-789 DOI: https://doi.org/10.1103/PhysRevB.37.785
Becke, A. D. J. Chem. Phys 1993, 98, 5648-5652 DOI: https://doi.org/10.1063/1.464913
Becke, A.D. J. Chem. Phys. 1996, 104, 1040-1046 DOI: http://dx.doi.org/10.1063/1.470829 DOI: https://doi.org/10.1063/1.470829
Hay, P. J.; Wadt, W. R. J. Chem. Phys. 1985, 82, 270-283 DOI: http://dx.doi.org/10.1063/1.448799 DOI: https://doi.org/10.1063/1.448799
Wadt, W. R.; Hay, P. J. J. Chem. Phys. 1985, 82, 284-298 DOI: http://dx.doi.org/10.1063/1.448800 DOI: https://doi.org/10.1063/1.448800
Hay, P. J.;Wadt, W. R. J. Chem. Phys. 1985, 82, 299-310 DOI: http://dx.doi.org/10.1063/1.448975 DOI: https://doi.org/10.1063/1.448975
Perrin, D. D. Stability Constants of Metal-ion Complexes. Part B. Organic Ligand IUPAC Chemical Data Series, No. 22. Pergamon Press, Oxford, 1979.
Arena, G.; Calì, R.; Contino, A.; Musumeci, A.; Musumeci, S.; Purrello, R. Inorg. Chim. Acta 1995, 237, 187-191 DOI: https://doi.org/10.1016/0020-1693(95)04655-S
Natsume, T.; Aizawa, S. I.; Hatano, K.; Funahashi, S. Dalton Trans. 1994, 2749-2753 DOI: https://doi.org/10.1039/DT9940002749
Buzás, N.; Gajda, T.; Nagy, L.; Kuzmann, E.; Vertes, A.; Burger, K. Inorg. Chim. Acta 1998, 274, 167- 176 DOI: https://doi.org/10.1016/S0020-1693(97)06044-1
De Stefano, C.; Foti C.; Gianguzza A.; Martino M.; Pellerito L.; Sammartano S. J. Chem. Eng. Data 1996, 41, 511-515 DOI: https://doi.org/10.1021/je9502915
Foti, C.; Gianguzza, A.; Millero, F.; Sammartano, S. Aquat. Geochem.1999, 5, 381-398. DOI: https://doi.org/10.1023/A:1009613712280
Rees, D. O. J. Mol. Biol. 1980, 141, 323-326 DOI: https://doi.org/10.1016/0022-2836(80)90184-9
Rogers, N. K.; Roore, G. R.; Strenberg, M. J. E. J. Mol. Biol. 1985, 182 , 613-616 DOI: https://doi.org/10.1016/0022-2836(85)90248-7
Sigel, H.; Martin, R. B.; Tribolet, R.; Haring, U. K.; Malini-Balakrishrann, R. Eur. J. Biochem. 1985, 152, 187-193 DOI: https://doi.org/10.1111/j.1432-1033.1985.tb09180.x
Åkerlöf, G.; Short, O. A. J. Am. Chem. Soc. 1936, 58, 1241-1243 DOI: https://doi.org/10.1021/ja01298a044
Shoukry, M. M.; El-Medani, S. M. Collect. Czech. Chem. Commun. 1997, 62, 1023-1028 DOI: https://doi.org/10.1135/cccc19971023
Mun, L. C.; Hapipah, M. A.; Shin, S. K. Appl. Organometal. Chem. 2012, 26, 310-319 DOI: https://doi.org/10.1002/aoc.2862
Al-Flaijj, O.; Shehata, M. R.; Mohamed, M. M. A.; Shoukry, M. M. Monatsh. Chem. 2001, 132, 349-366. DOI: https://doi.org/10.1007/s007060170 DOI: https://doi.org/10.1007/s007060170121
Nath, M.; Singh, H.; Eng, G.; Song. X. Inorg.

Downloads
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









