H3PW12O40 Anchored on the Three-Dimensional and Networked SBA-15 as an Efficient and Recyclable Catalyst for Mannich Reaction
DOI:
https://doi.org/10.29356/jmcs.v64i1.1034Keywords:
H3PW12O40, 3D-SBA-15, heterogeneous catalysis, Mannich reactionAbstract
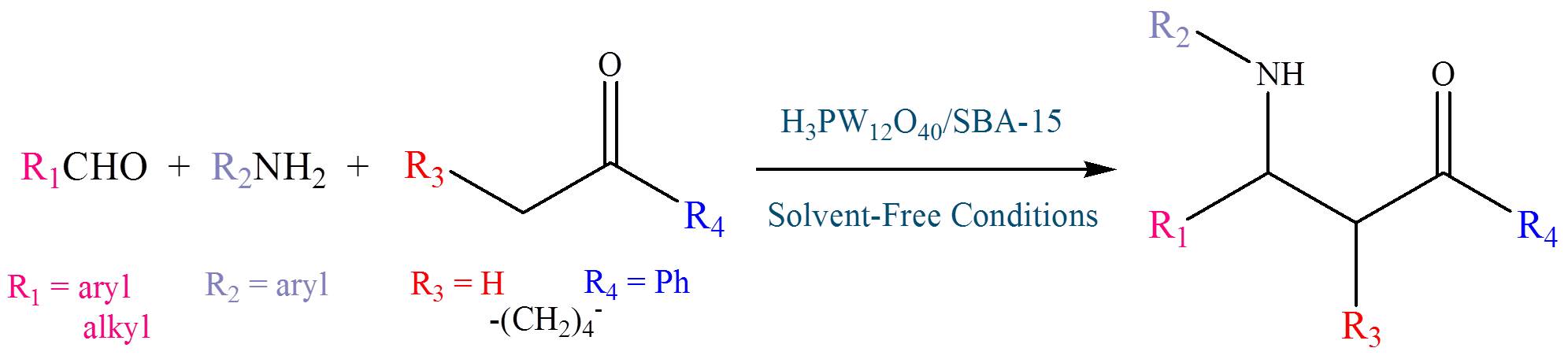
Abstract. The three-dimensional and networked SBA-15 (3D-SBA-15) supported phosphotungstic acid (PW) was used as heterogeneous catalyst for the one-pot three-components Mannich reaction at room temperature. The H3PW12O40/3D-SBA-15 catalyst was prepared using an impregnation method and confirmed by series of characterizations such as Fourier-transform infrared spectra (FT-IR), scanning electron microscope (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), N2 physisorption and thermogravimetric (TG) analysis. 50PW/3D-SBA-15 catalyst with H3PW12O40 loading of 50 wt% showed the highest yield of 93% in 1.8 h for the Mannich reaction of benzaldehyde, aniline and acetophenone under solvent-free condition. A series of β-aminoketone derivatives were synthesized successfully in the presence of this catalyst. In addition, H3PW12O40/3D-SBA-15 catalyst can be easily recovered and reused four times without significant decrease of the activity. This work provides an improved modification of the three-component Mannich reaction in terms of mild reaction conditions, clean reaction profiles, small quantity of catalyst and a simple workup procedure.
Resumen. El ácido fosfotungstico (PW) se soportó en sílice SBA-15 (3D-SBA-15) y se usó como catalizador heterogéneo en la reacción de Mannich en un solo paso de tres componentes a temperatura ambiente. El catalizador H3PW12O40/3D-SBA-15 se preparó mediante impregnación y se caracterizó por espectroscopia de infrarrojo (FT-IR), microscopia electrónica de barrido (SEM), microscopía electrónica de transmisión (TEM), difracción de rayos-X (XRD), fisisorción de N2 y análisis termogravimétrico (TG). El catalizador 50PW/3D-SBA-15, con una carga de H3PW12O40 del 50% en peso, mostró el rendimiento más alto del 93% en 1.8 h para la reacción de Mannich entre benzaldehído, anilina y acetofenona, sin disolvente. Se sintetizó una serie de derivados de β-aminocetona en presencia de este catalizador. Además, el catalizador H3PW12O40/3D-SBA-15 puede recuperarse fácilmente y reutilizarse cuatro veces sin pérdida significativa de la actividad. Este trabajo reporta una modificación de la reacción de Mannich de tres componentes bajo condiciones de reacción suaves, perfiles de reacción limpios, pequeña cantidad de catalizador y un procedimiento de tratamiento simple.
Downloads
References
Vaccaro, L. Beilstein J. Org. Chem. 2016, 12, 2763-2765.
Rafiee, E.; Eavani, S. Green Chem. 2011, 13, 2116.
Yang, H.; Dong, H.; Zhang, T.; Zhang, Q.; Zhang, G.; Wang, P.; Liu, Q. Catal. Lett. 2018, 149, 778-787.
Yang, L.; Xu, L.W.; Xia, C.G. Tetrahedron Lett. 2005, 46, 3279-3282.
Yan, X.; Chen, J.; Xue, Q.; Miele, P. Micropor. Mesopor. Mat. 2010, 135, 137-142.
Hussain, M.; Liu, J.; Zhang, Z.; Hu, M.; Li, Y.; Min, X. ChemistrySelect 2018, 3, 8787-8792.
Yeszhanov, A.B.; Mashentseva, A.A.; Korolkov, I.V.; Gorin, Y.G.; Kozlovskiy, A.L.; Zdorovets, M.V. Chem. Pap. 2018, 72, 3189-3194.
Pettignano, A.; Bernardi, L.; Fochi, M.; Geraci, L.; Robitzer, M.; Tanchoux, N.; Quignard, F. New J. Chem. 2015, 39, 4222-4226.
Sawant, D.P.; Justus, J.; Balasubramanian, V.V.; Ariga, K.; Srinivasu, P.; Velmathi, S.; Halligudi, S.B.; Vinu, A. Chem. 2008, 14, 3200-3212.
Okuhara, T.; Mizuno, N.; Misono, M. Appl. Catal. A-Gen. 2001, 222, 63-77.
Rafiee, E.; Joshaghani, M.; Eavani, S.; Rashidzadeh, S. Green Chem. 2008, 10, 982-989.
Sahoo, S.; Joseph, T.; Halligudi, S.B. J. Mol. Catal. A: Chem. 2006, 244, 179-182.
Zhang, Y.; Zhao, Y.; Xia, C. J. Mol. Catal. A: Chem. 2009, 306, 107-112.
Zhang, H.; Mifsud, M.; Tanaka, F.; Barbas Iii, C.F. J. Am. Chem. Soc. 2006, 128, 9630-9631.
Timofeeva, M.N. Appl. Catal. A-Gen 2003, 256, 19-35.
Wang, Y.; Satyavolu, N.S.R.; Lu, Y. Curr. Opin. Colloid Interface Sci. 2018, 38, 158-169.
Chen, G.; Zhang, X.; Guo, C.; Yuan, G. Russ. J. Phys. Chem. A 2010, 84, 2247-2253.
Hu, B.; Liu, H.; Tao, K.; Xiong, C.; Zhou, S. J. Phys. Chem. C 2013, 117, 26385-26395.
Dong, C.; Li, X.; Wang, A.; Chen, Y.; Liu, H. Catal. Commun. 2017, 100, 219-222.
Dan, H.; Dong, X.; Lu, X.; Ding, Y. J. Sol-Gel Sci. Technol. 2017, 81, 782-790.
Schwanke, A.; Favero, C.; Balzer, R.; Bernardo-Gusmão, K.; Pergher, S. J. Braz. Chem. Soc. 2017, 29,328-333.
Yang, Q.; Gu, F.; Tang, Y.; Zhang, H.; Liu, Q.; Zhong, Z.; Su, F. RSC Adv. 2015, 5, 26815-26822.
Zhou, Y.; Lin, W.G.; Yang, J.; Gao, L.; Lin, N.; Yang, J.Y.; Hou, Q.; Wang, Y.; Zhu, J.H. J. Colloid Interface Sci. 2011, 364, 594-604.
Liu, Q.; Tian, Y. Int. J. Hydrogen Energy 2017, 42, 12295-12300.
Cele, Z.E.D.; Pawar, S.A.; Naicker, T.; Maguire, G.E.M.; Arvidsson, P.I.; Kruger, H.G.; Govender, T. Eur. J. Org. Chem. 2014, 2014, 2253-2260.
Liu, Q.; Zhang, Z. Catal. Sci. Technol. 2019, 9, 4821-4834.
Wang, H.; Gao, X.; Wang, Y.; Wang, J.; Niu, X.; Deng, X. Ceram. Int. 2012, 38, 6931-6935.
Kooti, M.; Kooshki, F.; Nasiri, E.; Sedeh, A.N. J. Iran. Chem. Soc. 2018, 15, 943-953.
Armatas, G.S.; Katsoulidis, A.P.; Petrakis, D.E.; Pomonis, P.J. J. Mater. Chem. 2010, 20, 8631-8638.
Zhu, X.; Chen, C.; Yu, B.; Wu, Y.; Zhang, G.; Zhang, W.; Gao, Z. Catal. Sci. Technol. 2015, 5, 4346-4349.
Hussain, M.; Liu, J.; Zhang, Z.; Hu, M.; Li, Y.; Min, X. ChemistrySelect 2018, 3, 8787-8792.
Gawande, M.B.; Velhinho, A.; Nogueira, I.D.; Ghumman, C.A.A.; Teodoro, O.M.N.D.; Branco, P.S. RSC Adv. 2012, 2, 6144-6149.
Eftekhari-Sis, B.; Hashemi, M.M.; Farmad, F. Synth. Commun. 2009, 39, 4441-4453.
Azizi, N.; Torkiyan, L.; Saidi, M.R. Org. Lett. 2006, 8, 2079-2082.
Downloads
Additional Files
Published
Issue
Section
License
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.









